Colchicine derivative as well as preparation method and application thereof
A technology for colchicine and derivatives, which is applied in the field of colchicine derivatives and their preparation, can solve the problems of high cytotoxicity, achieve the effects of reducing cytotoxicity, maintaining anti-tumor activity, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Embodiment 1: the synthesis of structural formula (Ia-1) compound
[0032]
[0033] The synthetic route is as follows:
[0034]
[0035] Concrete synthetic steps are as follows:
[0036] To a solution of colchicine compound 1a (1 g, 2.6 mmol) in 10 mL of acetonitrile was sequentially added di-tert-butyl dicarbonate (2.3 g, 10.4 mmol), 4-dimethylaminopyridine (0.64 g, 5.2 mmol) and triethylamine (1.5mL, 10.4mmol), reflux for 4 hours. Add water to quench the reaction, first spin out most of the acetonitrile and then extract with ethyl acetate, the organic phase is sequentially washed with 1M HCl, saturated NaHCO 3 solution and washed with water, anhydrous Na 2 SO 4 After drying, column (dichloromethane / acetonitrile=4:1) gave the product 2a as a yellow solid (0.89 g, 68% isolated yield).
[0037] Sodium methoxide (200 mg, 3.6 mmol) was added to a solution of compound 2a (0.89 g, 1.8 mmol) in 10 mL of methanol, and reacted at room temperature for 0.5 hours. Add ...
Embodiment 2
[0040] Embodiment 2: the synthesis of structural formula (Ib-1) compound
[0041]
[0042] The synthetic route is as follows:
[0043]
[0044] Compound 4a (100 mg, 0.28 mmol) and compound 5b (124 mg, 0.34 mmol) were dissolved in 5 mL of N-methylpyrrolidone, then triethylamine (80 μL, 0.56 mmol) was added, and heated to 100°C for 1 hour. After cooling to room temperature, ethyl acetate was added, and H 2 O washed, anhydrous Na 2 SO 4 After drying, column (dichloromethane / methanol=20:1) gave product 6b (145 mg, 89% isolated yield) as a yellow solid.
[0045] Will K 2 CO 3 (345 mg, 2.5 mmol) in 1 mL of aqueous solution was added to compound 6b (145 mg, 0.25 mmol) in 3 mL of methanol solution, and reacted at room temperature for 0.5 hours. First spin out most of the methanol and then extract with dichloromethane, anhydrous Na 2 SO 4 After drying, compound 7b was spin-dried to obtain compound 7b, which was used directly without further purification because compound 7b ...
Embodiment 3
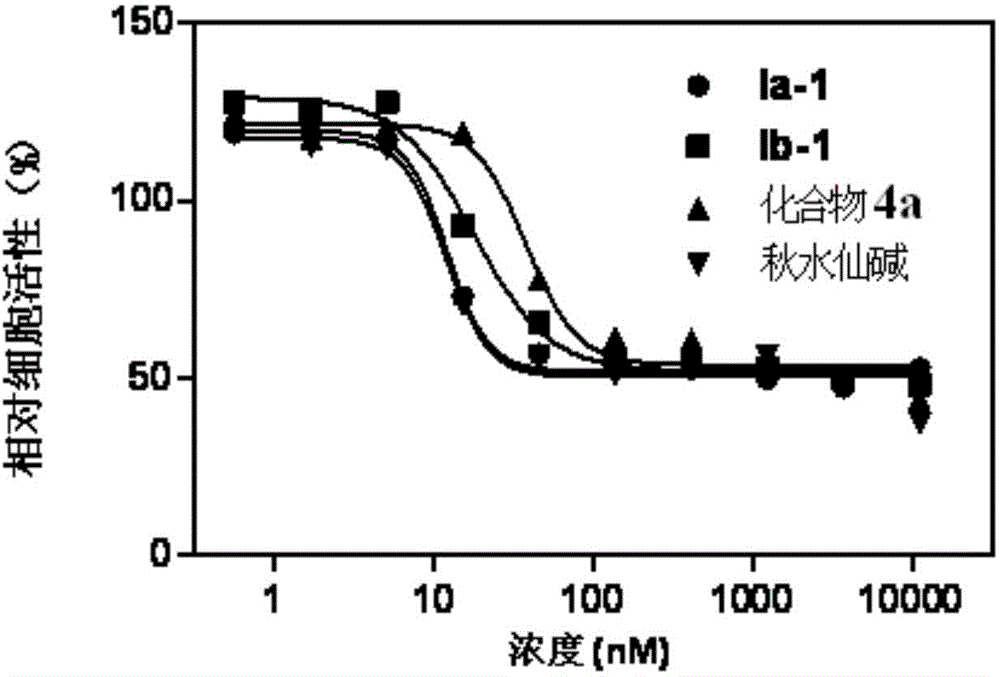
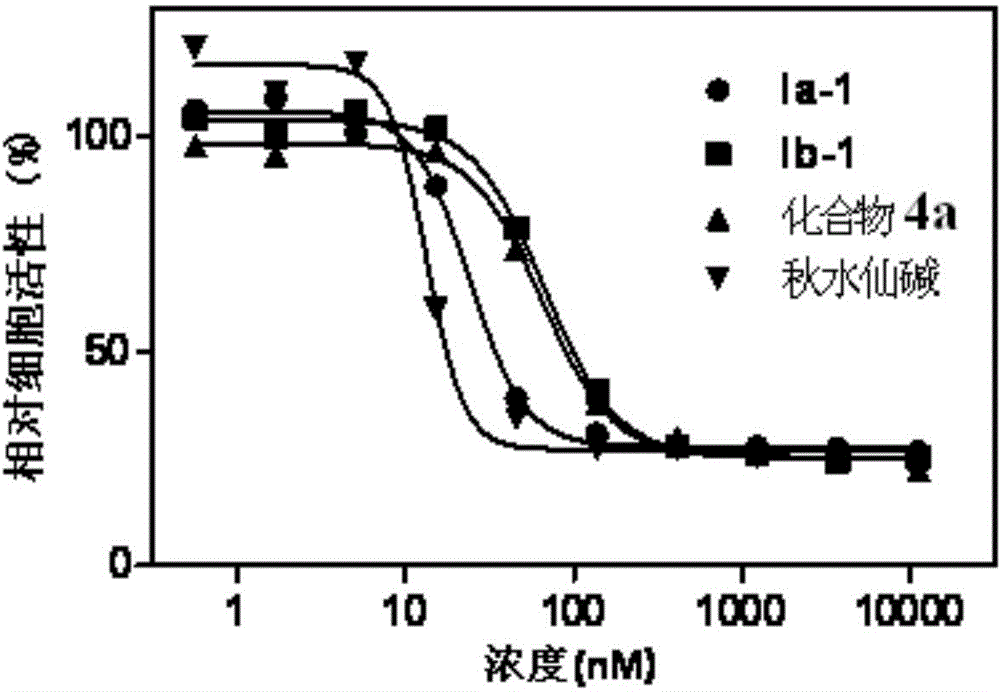
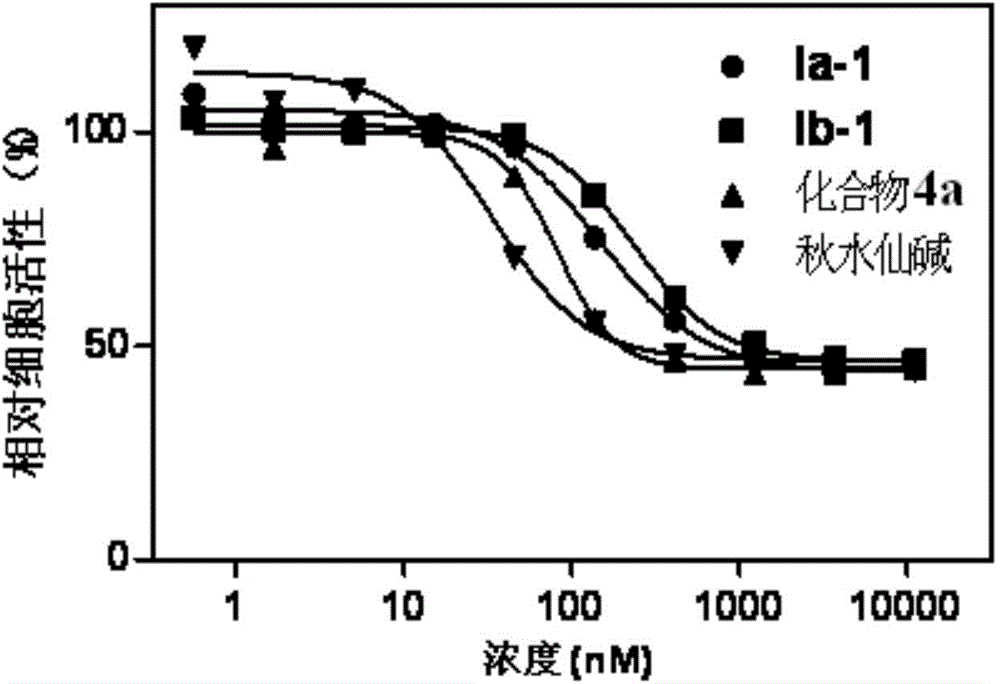
[0047] Embodiment 3: biological activity test
[0048] Test method: Add cells to a 96-well plate, about 10,000 cells per well, at 37 degrees, 5% CO 2 Adhere to the wall overnight under environmental conditions, remove the medium, and add fresh medium containing different concentrations of compounds. After 48 hours of incubation, MTS reagent (Promega) was added and read (490 nm, Perkin-Elmer Envision) two hours later.
[0049] Activity data:
[0050] The activity of colchicine and its derivatives on cancer cells and the toxicity test on normal cells are expressed by IC50 (nM) values, and the data results are shown in Table 1.
[0051] Table 1, IC50 values of colchicine and its derivatives on cancer cells and normal cells
[0052] compound
SKBR3
HEK293
3T3-L1
A
B
Ia-1
12.18
24
146.8
0.59
12.05
Ib-1
17.86
67.2
231.4
0.93
12.96
Compound 4a
37.94
64.58
81.84
0.59
2.16 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com