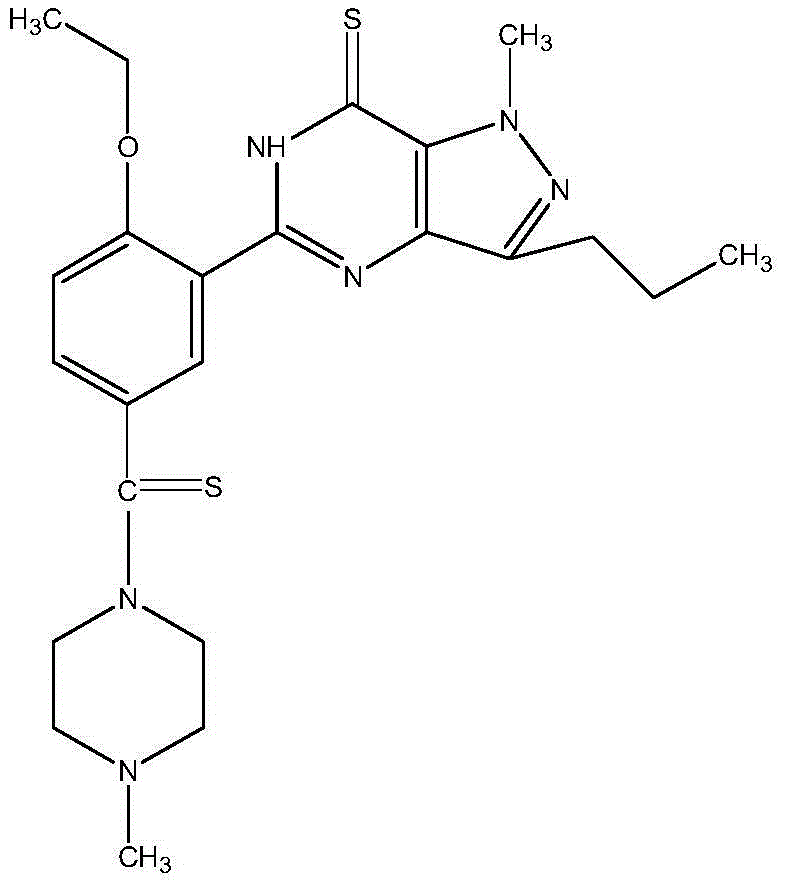
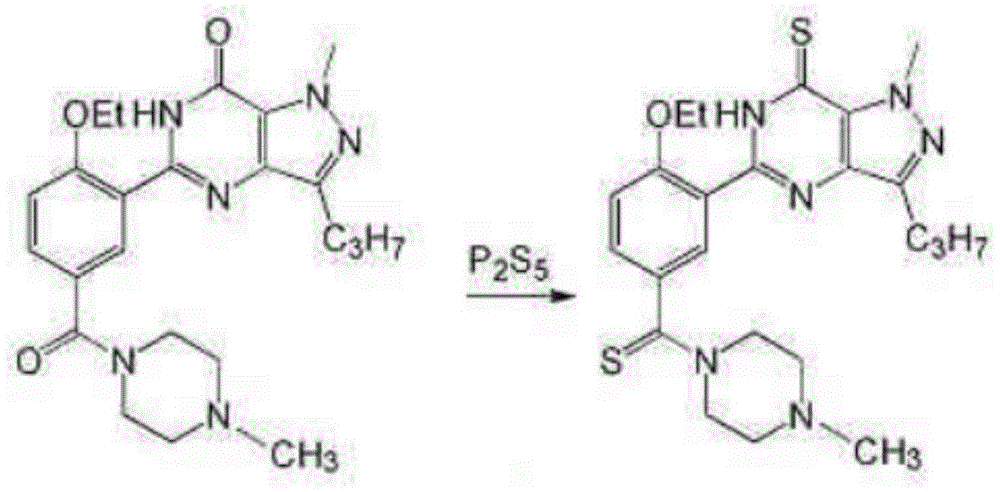
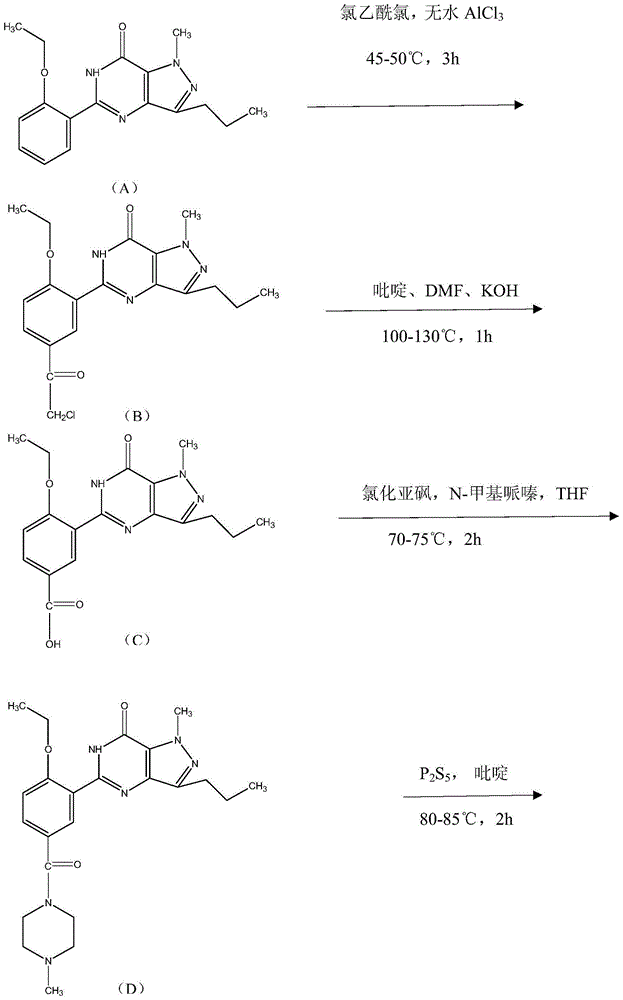
Synthesis method of sildenafil analog
A technology of sildenafil and a synthesis method, which is applied in the field of sildenafil analog synthesis, can solve the problems of unsuitability for industrial scale-up production, complicated operation and high production cost, and achieves reduction of total cost, simple operation and total income. high rate effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] A kind of synthetic method of sildenafil analogue, comprises the following steps:
[0035] (1) Compound (B): 5-[2-ethoxy-5-(chloromethyl-1-ylcarbonyl)]phenyl-1-methyl-3-n-propyl-1,6-dihydro Preparation of -7H-pyrazolo[4,3-d]pyrimidin-7-one
[0036] Put 180g (1.592mol) of chloroacetyl chloride into a dry 500ml reaction bottle, cool the reaction solution to below 20°C, add 122.4g (0.9175mol) of anhydrous aluminum trichloride in batches below 20°C, and stir for 10min . After the reaction was completed, the reaction solution was cooled with ice water to below 20°C, and 55.68g (0.1784mol) of compound (A) was put into the reaction flask in batches, and the feeding speed was controlled so that the internal temperature did not exceed 20°C. After the casting, the reaction was stirred at 45-50°C for 3h. After the reaction was completed, the reaction solution was added back into the ice-water mixture. After the solid material was completely precipitated, it was filtered, collec...
Embodiment 2
[0044] A kind of synthetic method of sildenafil analogue, comprises the following steps:
[0045] (1) Compound (B): 5-[2-ethoxy-5-(chloromethyl-1-ylcarbonyl)]phenyl-1-methyl-3-n-propyl-1,6-dihydro Preparation of -7H-pyrazolo[4,3-d]pyrimidin-7-one
[0046] Put 180g (1.592mol) of chloroacetyl chloride into a dry 500ml reaction bottle, cool the reaction solution to below 20°C, add 125g (0.9370mol) of anhydrous aluminum trichloride in batches below 20°C, and stir for 10min after the injection is complete. After the reaction was completed, the reaction solution was cooled with ice water to below 20°C, and 55.72g (0.1785mol) of compound (A) was put into the reaction flask in batches, and the feeding speed was controlled so that the internal temperature did not exceed 20°C. After the casting was completed, the reaction was stirred at 45-50°C for 4h. After the reaction was completed, the reaction solution was added back into the ice-water mixture. After the solid material was comple...
Embodiment 3
[0054] A kind of synthetic method of sildenafil analogue, comprises the following steps:
[0055] (1) Compound (B): 5-[2-ethoxy-5-(chloromethyl-1-ylcarbonyl)]phenyl-1-methyl-3-n-propyl-1,6-dihydro Preparation of -7H-pyrazolo[4,3-d]pyrimidin-7-one
[0056] Put 180g (1.592mol) of chloroacetyl chloride into a dry 500ml reaction bottle, cool the reaction solution to below 20°C, add 124.3g (0.9317mol) of anhydrous aluminum trichloride in batches below 20°C, and stir for 10min . After the reaction was completed, the reaction solution was cooled with ice water to below 20°C, and 55.86g (0.1789mol) of compound (A) was put into the reaction flask in batches, and the feeding speed was controlled so that the internal temperature did not exceed 20°C. After the casting, the reaction was stirred at 45-50°C for 2h. After the reaction was completed, the reaction solution was added back into the ice-water mixture. After the solid material was completely precipitated, it was filtered, collec...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com