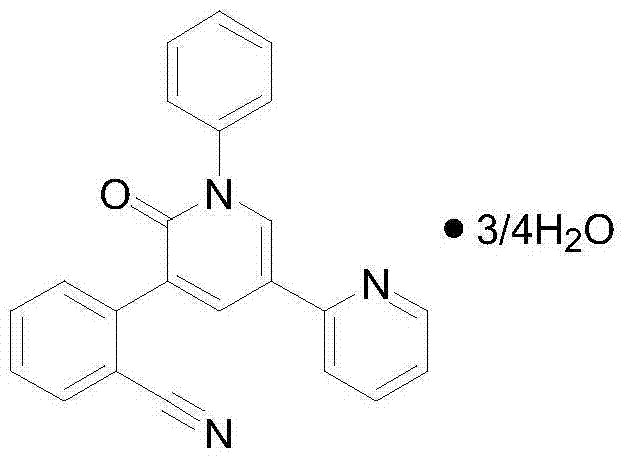
Perampanel freeze-dried oral disintegrating tablet and preparation method thereof
An orally disintegrating tablet and freeze-drying technology, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problems affecting patients' emotions, esophageal discomfort, and difficulties in taking coated tablets, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The dosage of each component in the preparation:
[0048]
[0049]
[0050] Add the skeleton proppant mannitol, binder gelatin, and freeze-drying protective agent dextran into water to fully dissolve, add perampanel, stir evenly, and dissolve to 0.2L;
[0051] Draw the liquid medicine and inject 0.2ml into each blister model;
[0052] Place the mold in a -50°C environment for quick freezing;
[0053] Put the solid liquid medicine into the freeze-drying box, vacuumize at -30℃ low temperature environment, and keep the vacuum for 3 hours at a vacuum degree of 10-20Pa;
[0054] Raise the temperature to -10℃~-15℃ and keep it for 3 hours;
[0055] Raise the temperature to 5°C-15°C and keep it for 1 hour;
[0056] Raise the temperature to 20°C-30°C and keep it for 1 hour;
[0057] Out of the box, laminating, cutting, and printing the batch number.
Embodiment 2
[0059] The dosage of each component in the preparation:
[0060]
[0061] Add the skeleton proppant mannitol, binder gelatin, freeze-drying protective agent dextran, and flavoring agent aspartame into water to fully dissolve, add perampanel, stir well, and dissolve to 0.2L;
[0062] Draw the liquid medicine and inject 0.2ml into each blister model;
[0063] Place the mold in a -40°C environment for quick freezing;
[0064] Put the solid liquid medicine into the freeze-drying box, vacuumize at -20 ℃ low temperature environment, vacuum degree 10-20Pa, keep for 3 hours;
[0065] Raise the temperature to -10℃~-15℃ and keep it for 4 hours;
[0066] Raise the temperature to 5°C-15°C and keep it for 2 hours;
[0067] Raise the temperature to 20°C-30°C and keep it for 2 hours;
[0068] Out of the box, laminating, cutting, and printing the batch number.
Embodiment 3
[0070] The dosage of each component in the preparation:
[0071]
[0072] Add the skeleton proppant mannitol, binder hydrolyzed gelatin, freeze-drying protective agent dextran, and flavoring agent sucralose into water to fully dissolve, add perampanel, stir evenly, and settle to 0.2L;
[0073] Draw the liquid medicine and inject 0.2ml into each blister model;
[0074] Place the mold in a -60°C environment for quick freezing;
[0075] Put the solid liquid medicine into the freeze-drying box, vacuumize at -50℃ low temperature environment, the vacuum degree is 10-20Pa, keep for 3 hours;
[0076] Raise the temperature to -10℃~-15℃ and keep it for 4 hours;
[0077] Raise the temperature to 5°C-15°C and keep it for 1 hour;
[0078] Raise the temperature to 20°C-30°C and keep it for 2 hours;
[0079] Out of the box, laminating, cutting, and printing the batch number.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com