Novel Mg-Li-Al-Ti hydrogen storage material and preparing method thereof
A hydrogen storage material and a new type of technology, applied in the production of hydrogen, etc., can solve the problems of high requirements for hydrogen absorption conditions, great influence of dehydrogenation temperature, unstable catalytic performance of catalysts, etc., and achieve reduced reversible hydrogen absorption conditions and high capacity Hydrogen storage performance and the effect of improving kinetic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] A preparation method of a novel Mg-Li-Al-Ti hydrogen storage material, comprising the steps of:
[0016] Step 1, take 0.8855g LiH and 2.1149gLiAlH 4 , put into a ball mill jar with 24 steel balls, and use mechanical ball milling method to mix evenly. Under the condition of inert gas, the rotating speed is 270rpm, and the ball milling time is 8h. After ball milling, it was taken out in the glove box to obtain Li 3 H 6 ;
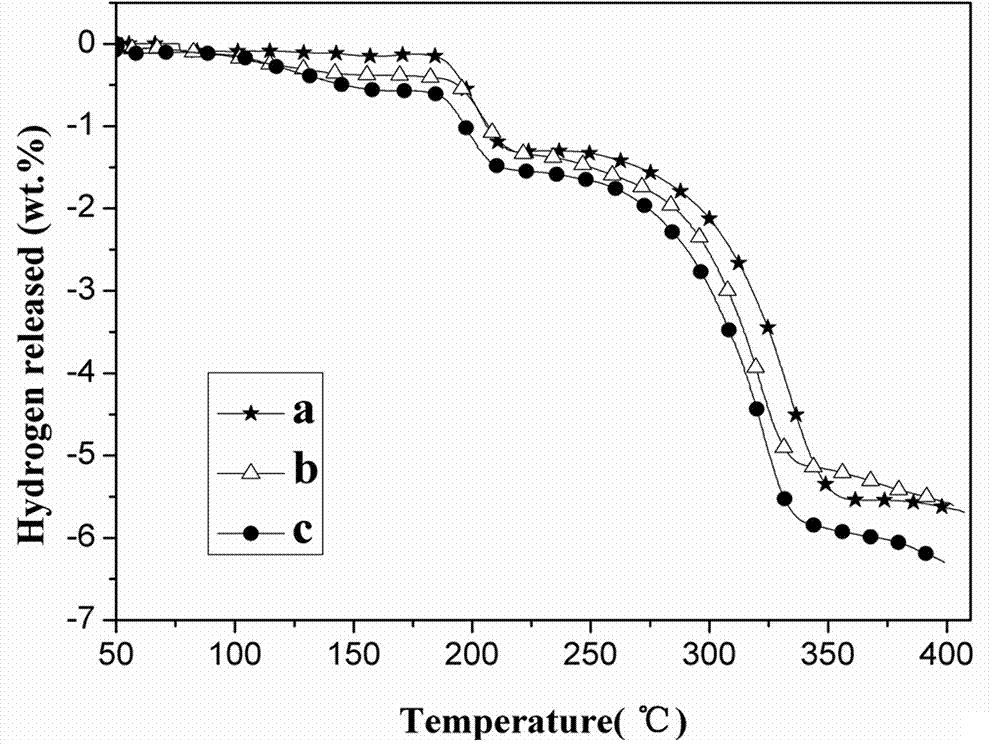
[0017] Step 2, take samples according to the compositions in Table 1, put them into ball milling jars equipped with 24 steel balls, and mix them evenly by mechanical ball milling. Under inert gas conditions, the rotating speed is 270rpm, and the ball milling time is 2h, and the samples are obtained a (66.2wt.% MgH 2 -33.8wt.% Li 3 H 6 ), b (58.8 wt.% MgH 2 -27.2 wt.% Li 3 H 6 -8 wt.% AlCl 3 -6 wt.% Ti) and c (53 wt.% MgH 2 -27 wt.% Li 3 H 6 - 14 wt.% AlCl 3 -6 wt.% Ti).
[0018] Table 1 Composition of three samples
[0019]
[0020] P...
Embodiment 2
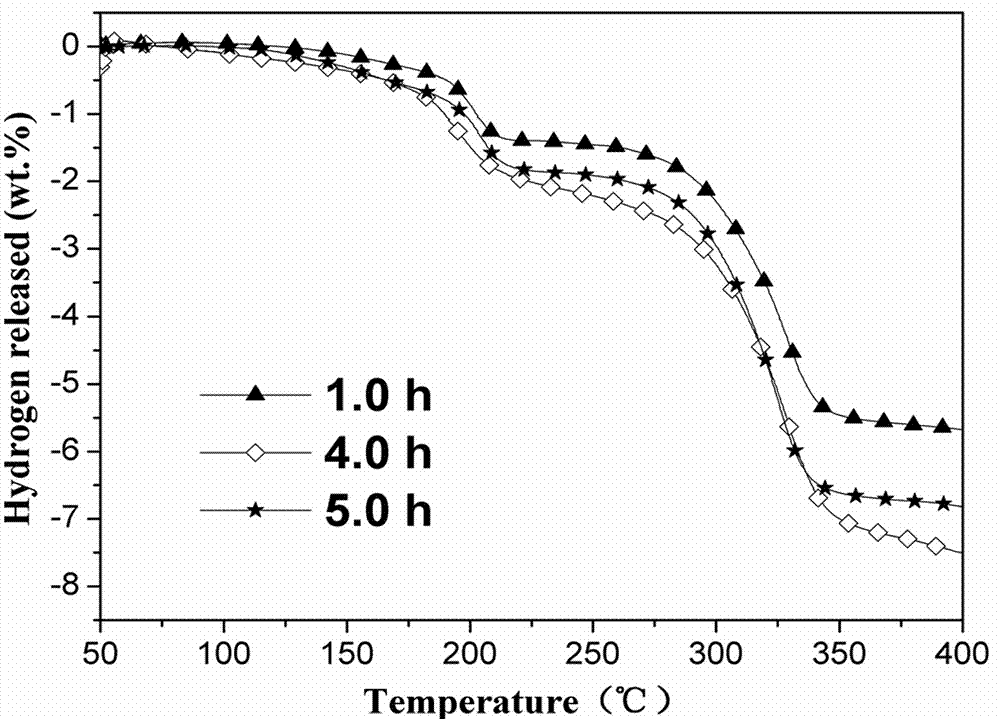
[0022] According to the composition of sample c, under the condition of other conditions unchanged, only change the ball milling time (1 h, 4 h and 5 h), prepare three composite systems with different ball milling time, and investigate the effect of ball milling time on the hydrogen production performance of the system influences.
[0023] From figure 2It can be seen from the dehydrogenation curves that the initial dehydrogenation temperature of ball milling for 1 h and 5 h is about 100 °C, but when the ball milling time is 4 h, the initial dehydrogenation temperature drops to about 58 °C, which is higher than that of 1 h and 5 h The initial dehydrogenation temperature of the composite system decreased by nearly 40 °C; and the total dehydrogenation amount (heated to 400 °C) was the largest when ball milled for 4 h, reaching 7.2 wt.%. The results show that the dehydrogenation amount will be reduced if the ball milling time is too long or too short.
Embodiment 3
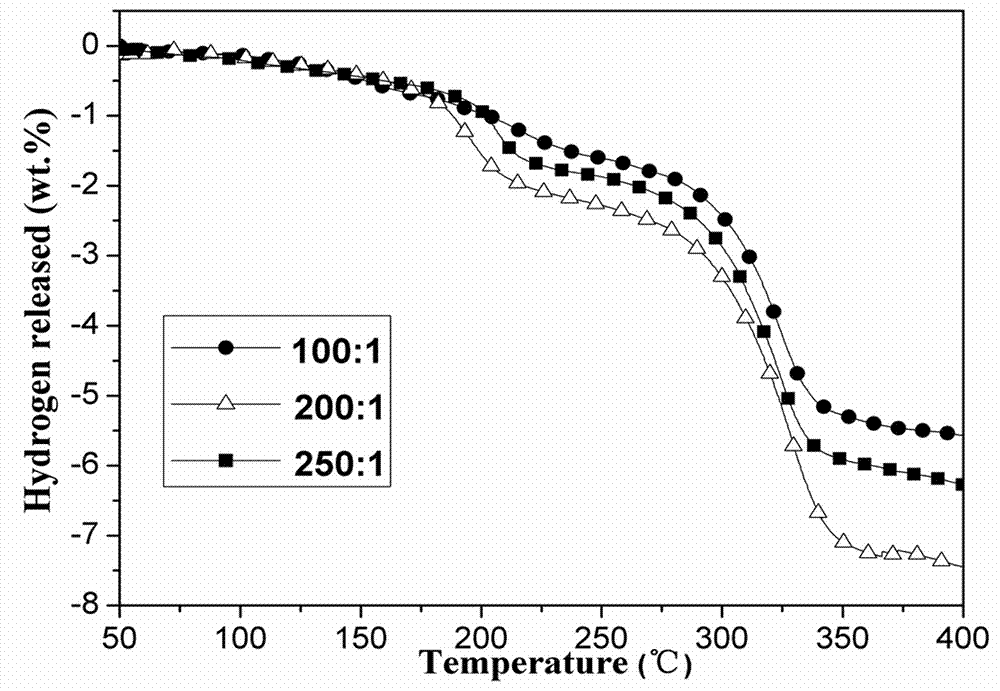
[0025] According to the composition of sample c, the ball milling time is 4 h. Under the condition of other conditions unchanged, only the ball-to-material ratio in the ball milling condition is changed [100:1 (sample Ⅰ), 200:1 (sample Ⅱ), 250:1 (Sample Ⅲ)] to investigate the effect of ball-to-material ratio on the hydrogen production performance of the system.
[0026] From image 3 It can be seen from the dehydrogenation curves that the increase of the ball-to-material ratio has little effect on the initial dehydrogenation temperature (the initial dehydrogenation temperatures of samples I, II, and III are all around 58°C), but for the total dehydrogenation temperature of 400°C Quantity matters. It can be seen from the figure that when the ball-to-material ratio is 100:1, the total dehydrogenation amount is 5.2wt.%; when the ball-to-material ratio is increased to 200:1, the total dehydrogenation amount increases to 7.2wt.%. Continue to increase the ball-to-material ratio Wh...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com