A kind of biological glass granular material and its preparation process
A bioglass and particle material technology, applied in cosmetic preparations, cosmetic preparations, dentistry, etc., can solve the problems of associated caries, affecting clinical desensitization efficiency, limited particle residual rate, etc., and achieve the effect of reducing sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] 1) Dissolve 2.0ml of concentrated nitric acid and 13.192ml of ethyl orthosilicate in 40ml of deionized water in turn, stir at room temperature for 30 minutes, and set aside;
[0057] 2) Add 2.859g of boric acid, 0.055g of sodium nitrate, 3.000g of potassium nitrate, 0.120g of copper nitrate, and 0.116g of zinc nitrate into the solution of step 1) in sequence, and add the two adjacent salts at an interval of 10 minutes, and stir at room temperature 10 minutes, spare;
[0058] 3) Add 12.651g of calcium nitrate, 0.286g of ammonium phosphate and 0.4g of citric acid to the solution in step 2), and stir it thoroughly for 10 minutes, then leave it to stand and age at 35°C for 72 hours, and set aside;
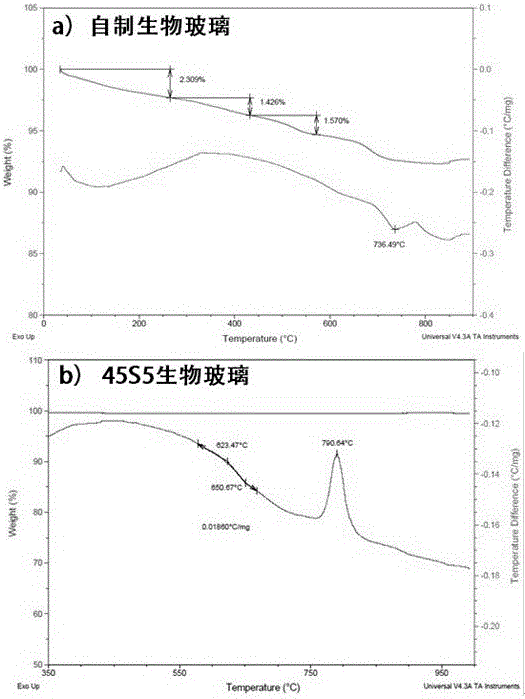
[0059] 4) Drying the aged gel in 3) at 120° C. for 24 hours, and then calcining at 620° C. for 90 minutes to obtain a bioglass ultrafine powder material. After determination, the contents of each oxide are: CaO 30%, SiO 2 38%,P 2 o 5 1.0%, B 2 o 3 16%, CuO 0.4%, ZnO 0.4%, ...
Embodiment 2
[0063] 1) Dissolve 1.6ml of concentrated nitric acid and 12.150ml of ethyl orthosilicate in 40ml of deionized water in turn, stir at room temperature for 60 minutes, and set aside;
[0064] 2) Add 2.680g of boric acid, 0.384g of sodium nitrate, 3.868g of potassium nitrate, 0.211g of copper nitrate, and 0.029g of zinc nitrate into the solution of step 1) in sequence, and add the two adjacent salts at an interval of 30 minutes, and stir at room temperature 30 minutes, spare;
[0065] 3) Add 11.870 g of calcium nitrate, 0.515 g of ammonium phosphate and 0.32 g of citric acid to the solution in step 2), and stir thoroughly for 45 minutes, then leave it to stand and age at 60° C. for 72 hours, and set aside;
[0066] 4) Drying the aged gel in 3) at 180° C. for 6 hours, and then calcining at 750° C. for 60 minutes to obtain a bioglass ultrafine powder material. After determination, the mass content of each oxide is: CaO 28%, SiO 2 35%,P 2 o 5 1.8%, B 2 o 3 15%, CuO 0.7%, ZnO 0...
Embodiment 3
[0068] 1) Dissolve 1.8ml of concentrated nitric acid and 12.150ml of ethyl orthosilicate in 40ml of deionized water in turn, stir at room temperature for 45 minutes, and set aside;
[0069] 2) Add 3.216g of boric acid, 1.096g of sodium nitrate, 3.868g of potassium nitrate, 0.181g of copper nitrate, and 0.058g of zinc nitrate into the solution of step 1) in sequence, and add the two adjacent salts at an interval of 15 minutes, and stir at room temperature 12 minutes, spare;
[0070] 3) Add 9.446g of calcium nitrate, 0.515g of ammonium phosphate and 0.15g of citric acid to the solution in step 2), and then fully stir for 15 minutes, then stand and age at 60°C for 48 hours, and set aside;
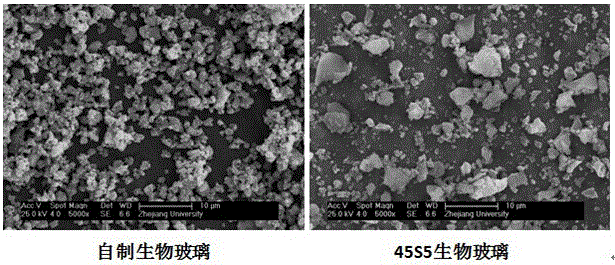
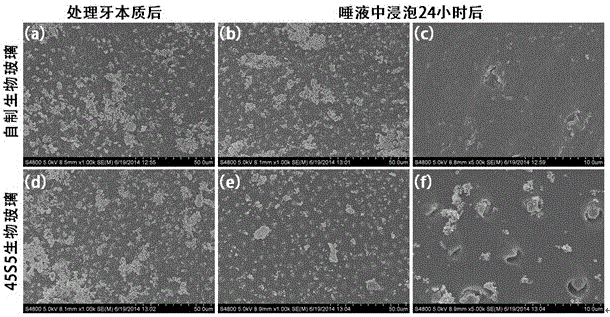
[0071] 4) Dry the aged gel in 3) at 150°C for 18 hours, and then calcinate at 650°C for 120 minutes to obtain a bioglass ultrafine particle powder material, and the particle size of the bioglass particles is between 250 and 1680 nanometers Between, the mass content of each oxide is: CaO 22.4%...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com