Stable CTLA4Ig protein preparation
A preparation and stable technology, applied in the field of CTLA4 molecular preparation, can solve the problems of reduced bioavailability of active protein, changes in pharmacokinetics, unwanted immunogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Example 1 The impact of the molecular weight of PVP on the soluble CTLA4 molecular protein preparation
[0021] The stability of soluble CTLA4 molecular protein preparations containing PVP with different molecular weights was studied at 25°C. The formulations contained in addition to PVP other excipients according to the details given in Table 1.
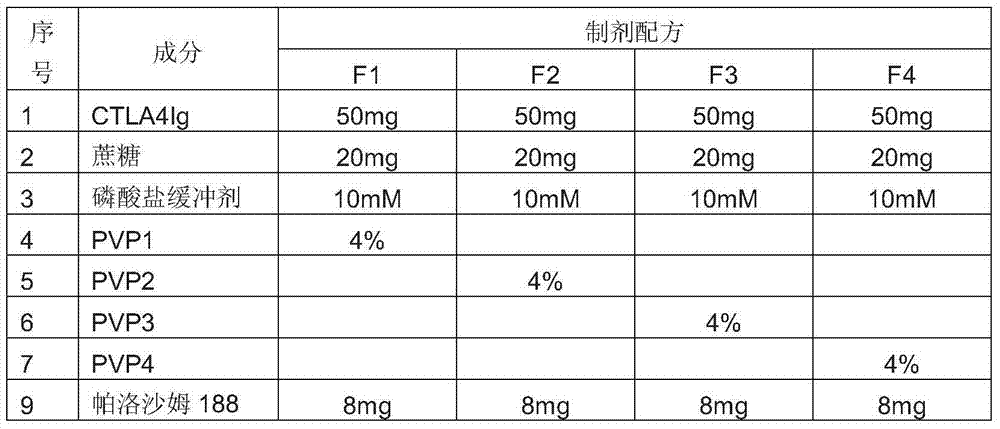
[0022] Table 1: Soluble CTLA4 molecular protein preparations containing PVP with different molecular weights
[0023]
[0024] Remarks: In the table, PVP1 (weight average molecular weight 44K-54K Daltons); PVP2 (weight average molecular weight 27K-32K Daltons); PVP3 (weight average molecular weight 2K-3K Daltons); PVP4 (weight average molecular weight 1M- 1.5M Dalton)
[0025] The above sample preparations were incubated at 25°C for one month and then analyzed by SDS-PAGE. SDS-PAGE is used as an analytical technique to separate free and high molecular weight species from native proteins according to their molecular weig...
Embodiment 2
[0033] Example 2 Effect of PVP Content on Soluble CTLA4 Molecular Protein Preparation
[0034] Formulations of CTLA4 molecules containing different amounts of low molecular weight PVP were investigated. The formulations contained, in addition to PVP, other excipients according to the details given in Table 3.
[0035] Table 3: CTLA4Ig preparation ingredient table
[0036]
[0037] CTLA4 molecular protein preparations with different contents of low molecular weight PVP as given in Table 3 were charged at 50 °C for 3 days, and then analyzed by SE-HPLC and differential scanning calorimetry (DSC).
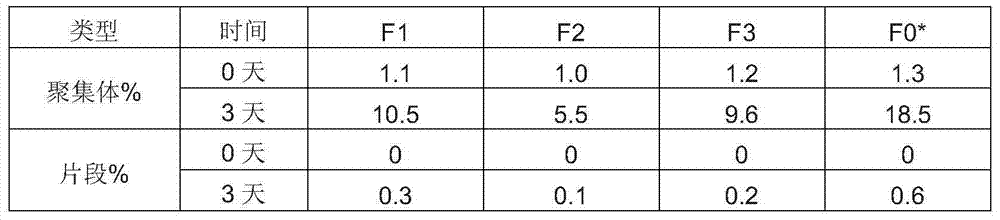
[0038] Table 4: Effects of different contents of low molecular weight PVP on formulation stability
[0039]
[0040] The results showed that the addition of 1% to 20% low molecular weight PVP could better inhibit the aggregation and fragmentation of high-concentration proteins. A more preferred low molecular weight PVP content ranges from 1% to 4% PVP2.
Embodiment 3
[0041] Example 3 Effect of Sugar on Soluble CTLA4 Molecular Protein Preparation
[0042] The effect of different levels of sugar on the stability of CTLA4Ig formulations was evaluated. We placed the samples at -20°C, 8°C and 25°C under the conditions of 60% humidity and detected the aggregation and fragmentation of proteins in CTLA4Ig preparations by SE-HPLC at different time points.
[0043] Table 5: Soluble CTLA4 molecular protein formulation formula table
[0044] CTLA4Ig
150mg / ml
Palosham 188
6mg / ml
Phosphate buffer
10mM
Pvp2
4%
See Table 8 for details
[0045] Table 6: Effect of sugar on the stability of soluble CTLA4 molecular protein preparations
[0046]
[0047] It can be seen from Table 6 that sugar has a great influence on the stability of CTLA4Ig preparations. Preferably, when the weight ratio of sucrose:CTLA4Ig is 170:125, the stability is the best.
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com