ALD and application thereof as EV71 virus and CAV16 virus inhibitor
A technology of CAV16 and EV71, applied in the field of ALD and its use as an inhibitor of EV71 virus and CAV16 virus, can solve the problems such as the listing of specific antiviral drugs for hand, foot and mouth disease
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
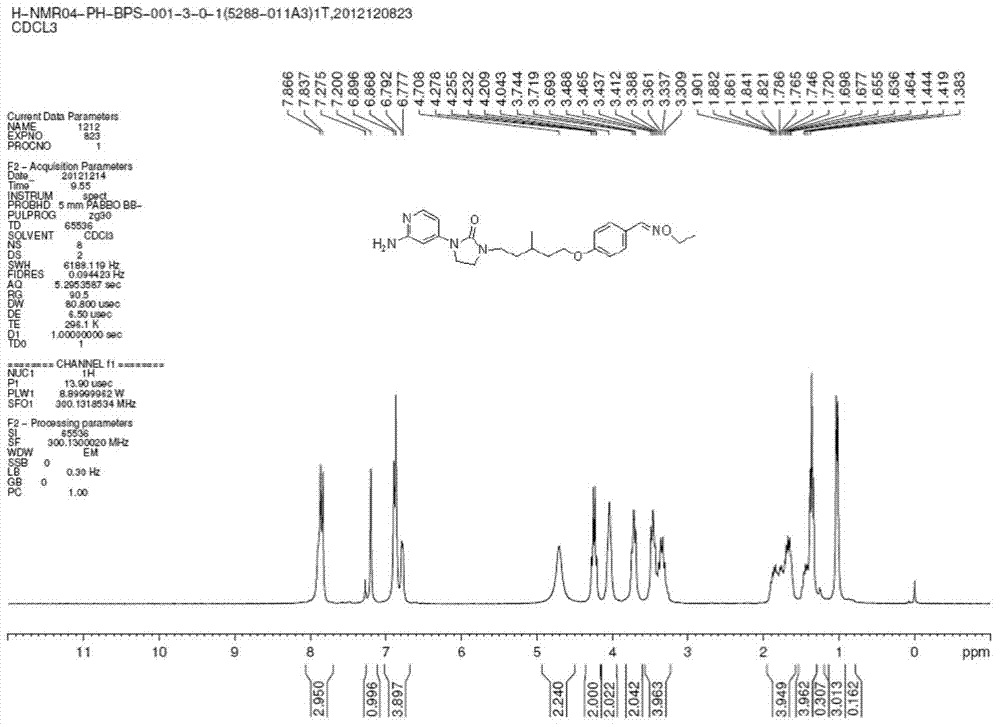
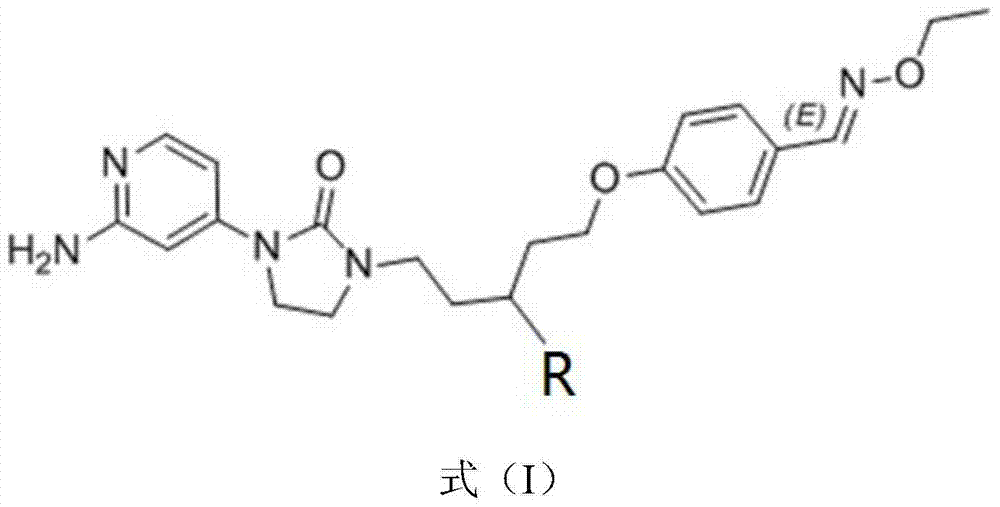
[0027] Example 1: R is -CH 3 Synthesis of the compound of the present invention:
[0028] (1) Add 4-bromopyridin-2-amine (5g, 28.90mmol), di-tert-butyl carbonate ((Boc) 2 O) (6.8g, 31.16mmol), 4-dimethylaminopyridine (0.32g), tert-Butanol (70mL). Stir overnight at room temperature and concentrate in vacuo. The resulting mixture was washed with 30 ml of n-hexane / ether (hexane / ether) with a volume ratio of 5:1 to obtain N-tert-butoxycarbonyl-4-bromo-pyridine-2-amine (tert-butyl N-(4-bromopyridin-2-yl)carbamate) (4.5g, 57%), a white solid.
[0029] (2) Under nitrogen, add tert-butyl-N-(4-bromopyridin-2-yl) carbonate (that is, the N-tert-butoxycarbonyl-4-prepared in step (1)) into a 50 ml round bottom flask. Bromo-pyridine-2-amine) (820mg, 3.00mmol), 1-(5-[4-[(ethoxyimine)methyl]phenoxy]-3-methylpentyl)-imidazoline- 2-ketone (1-(5-[4-[(ethoxyimino)methyl]phenoxy]-3-methylpentyl)imidazolidin-2-one) (500mg, 1.50mmol), Johnphos (2-(di-tert-butylphosphine) Benzene) (45mg), Pd(dba) 2 (38...
Embodiment 2
[0065] Example 2: The inhibitor compound prepared in Example 1 inhibits the activity of five hand-foot-mouth disease-causing viruses EV71(A), EV71(B), EV71(C), CAV16(A) and CAV16(B) infecting cells Determination
[0066] The inhibitor compound prepared in Example 1 was dissolved in 95% DMSO, prepared as a solution with a concentration of 10 mM, and stored at 4°C for later use.
[0067] According to experimental step 6 (inhibitor inhibits virus infection IC 50 The contents described in the determination of the value) were performed to test the protective effects of the inhibitor compound on the two types of cells (RD cells and Vero cells) under the above five virus infections. According to the method in experiment step 5, observe the corresponding inhibitor concentration when half of the cells have lesions, and obtain the IC50 value. The final measured results are shown in Table 2:
[0068] Table 2
[0069]
[0070]
[0071] It can be seen from the above experiments that the compound...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com