Application of cinchona alkaloid derivatives as cytotoxic compounds
A technology for cinchona alkaloids and drugs, which is applied in the directions of active ingredients of heterocyclic compounds, drug combinations, and medical preparations containing active ingredients, etc., can solve the problems of difficult, expensive and ineffective cancer treatment.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
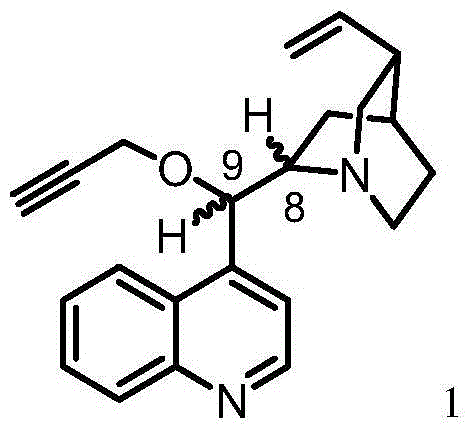
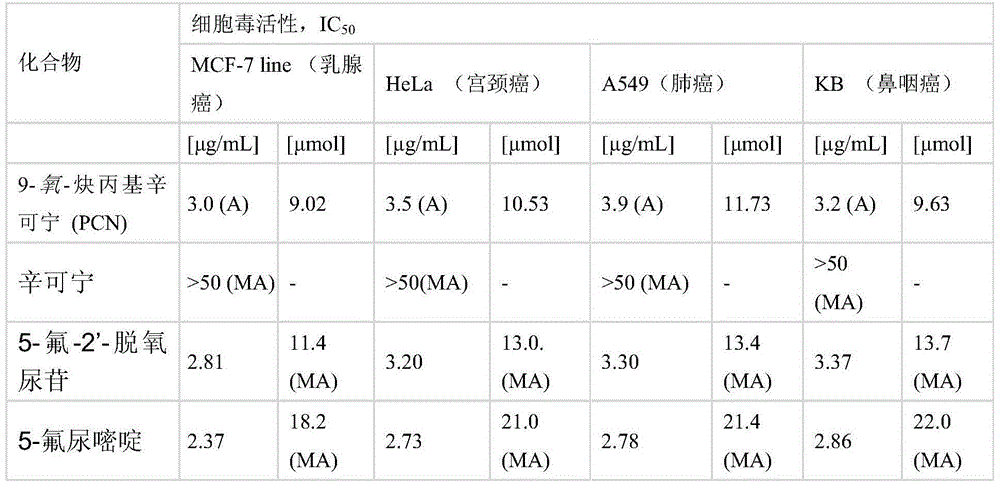
[0040] Cinchonine (883 mg, 3 mmol) was dissolved in anhydrous DMF (12 mL), and the solution was then placed in an ice bath. The mixture was cooled to about 5°C and sodium hydride (50% NaH in mineral oil, 300 mg, 2 equiv) was added in portions over 0.5 h. The solution was stirred for 2 hours and an 80% solution of propargyl bromide in toluene (0.42 mL, 3.75 mmol, 1.25 equiv) was added using a syringe. The reaction mixture was allowed to stand overnight at room temperature. Next, dichloromethane (50 ml) was added to the reaction mixture, and the organic solution was washed successively with saturated NaCl solution (30 mL) and distilled water (30 mL). The organic layer was dried with anhydrous magnesium sulfate, then, the desiccant was filtered off, and the solvent was evaporated using a vacuum evaporator, keeping the temperature of the water bath in the range of 40-45°C. The crude product 9-oxo-propargyl cinchonine (PCN) was purified on a column packed with silica gel (60H, 0....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com