A kit to test the effectiveness of oxaliplatin for colorectal cancer
A colorectal cancer and oxaliplatin technology, applied in the field of kits for detecting the effectiveness of oxaliplatin for colorectal cancer, can solve the problems of lack of clinical verification of oxaliplatin sensitivity and drug resistance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
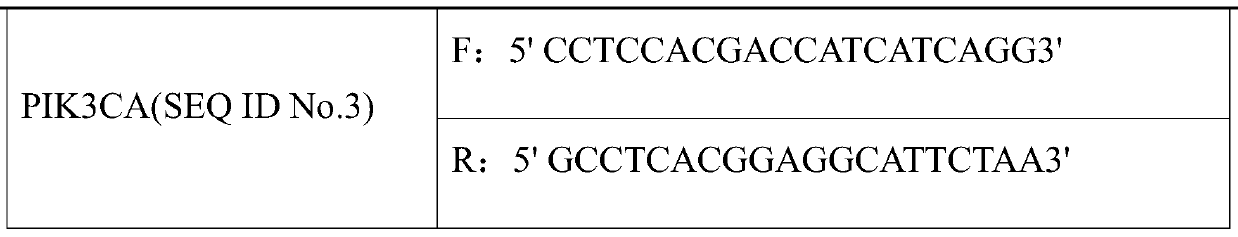
[0017] The kit includes: a real-time fluorescence quantitative PCR amplification system composed of 2×SYBR Green qPCR Mix, ultrapure water and two pairs of qPCR detection primers (see Table 1).
[0018] Table 1 Three pairs of qPCR detection primers
[0019]
[0020]
[0021] For the real-time fluorescent quantitative PCR amplification system, the final concentration of each 20 μL reaction system is: forward and reverse primers 4uM each, 2×SYBR Green qPCR Mix 10 μL, and ultrapure water to make up to 20uL.
[0022] The mM and μM are units of molar concentration, referring to the number of moles of solute per liter of solution.
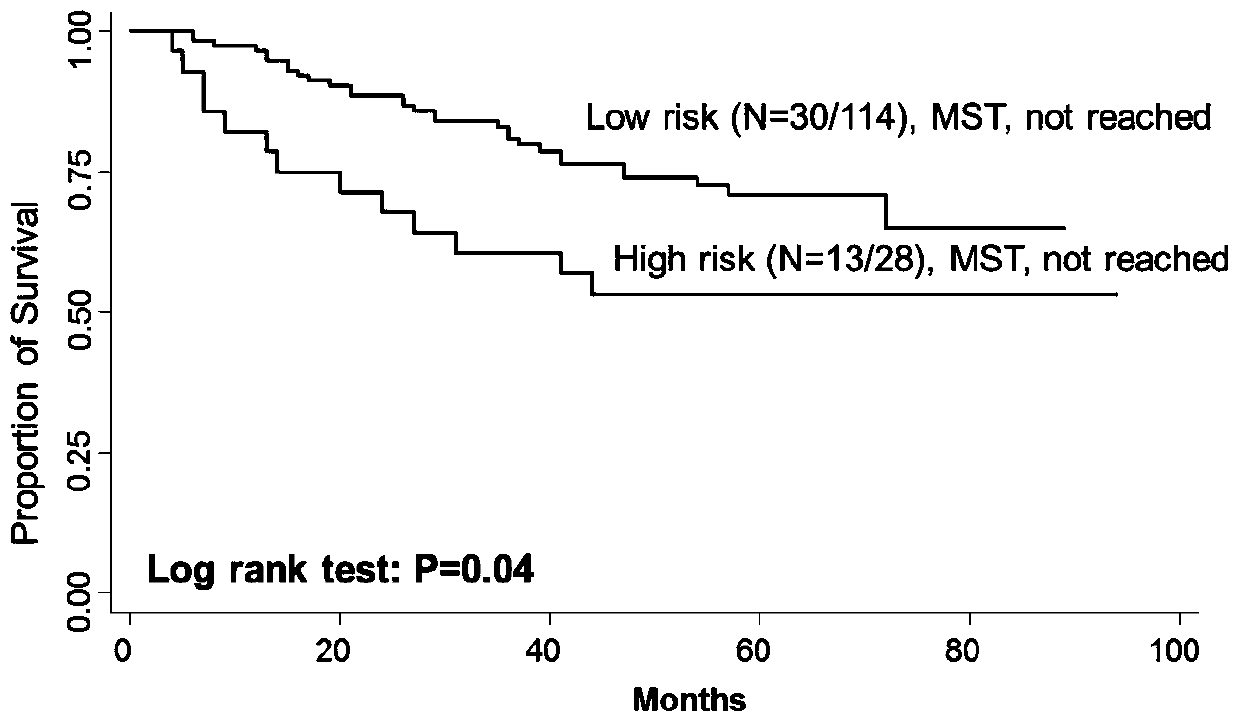
[0023] The present invention detects the change of MAP4K1, PIK3CA gene copy number and predicts the method for rectal cancer chemotherapy curative effect, mainly comprises the following steps:
[0024] (1) Collect surgically resected cancer tissue samples from patients with colorectal cancer and embed them in paraffin, and follow up the recurrence...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com