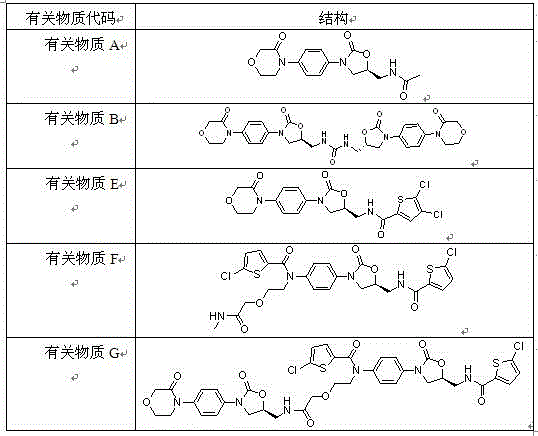
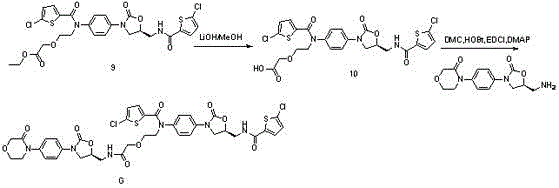
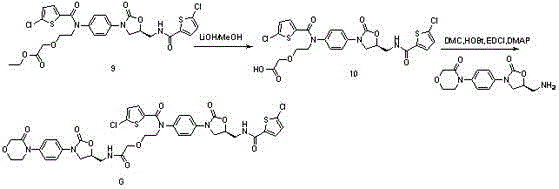
Synthetic methods of related substances F and G of rivaroxaban
A related substance, rivaroxaban technology, applied in the direction of organic chemistry, etc., can solve the problems of lack of impurities and cumbersome synthesis methods of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0058] Add (S)-5-chloro-N-({2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1 , 3-oxazolidin-5-yl}methyl)-2-carboxamide. In 100 mL aqueous solution of 16 g of hydrochloride, react at room temperature for 2 hours. Concentrate to remove water in the reaction solution, wash with 30 mL of methanol, filter, concentrate the filtrate, and dry to obtain (S)-5-chloro-N-( {2-oxo-3-[ 4-( 3-oxo- 4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-carboxamide, under the protection of argon at room temperature, add 4.5 mL of anhydrous triethylamine to (S ) -5-chloro-N-({2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl )-2-carboxamide in 600 mL of anhydrous tetrahydrofuran suspension, and then 5 mL of acetyl chloride was added dropwise, and reacted at room temperature for 2 hours. TCL (EA / MeOH=10:1) tracking shows that some raw materials still exist. Anhydrous 2.2 mL of triethylamine and 2.2 mL of acetyl chloride were added successively to continue the reaction for 2 hours. TCL ...
Embodiment 2
[0062] Add (S)-5-chloro-N-({2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1 , 3-oxazolidin-5-yl}methyl)-2-carboxamide. In 1.3mL aqueous solution of 180 g of hydrochloride, react at room temperature for 2.5 hours. Concentrate to remove water in the reaction solution, wash with 500 mL methanol, filter, concentrate the filtrate, and dry to obtain (S)-5-chloro-N-( {2-oxo-3-[ 4-( 3-oxo- 4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-carboxamide, under the protection of argon at room temperature, add 500 mL of anhydrous triethylamine to (S ) -5-chloro-N-({2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl )-2-carboxamide in 8.0L of anhydrous tetrahydrofuran suspension, and then 70 mL of acetyl chloride was added dropwise, and reacted at room temperature for 2 hours. TCL (EA / MeOH=10:1) tracking shows that some raw materials still exist. Then add anhydrous 25mL triethylamine and 25mL acetyl chloride to continue the reaction for 2 hours, TCL (EA / MeOH=10 / 1) monitoring,...
Embodiment 3
[0064] Add (S)-5-chloro-N-({2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1 , 3-oxazolidin-5-yl}methyl)-2-carboxamide. In 1.2 mL aqueous solution of 160 g of hydrochloride, react at room temperature for 2 hours. Concentrate to remove water in the reaction solution, then wash with 400 mL of methanol, filter, concentrate the filtrate, and dry to obtain (S)-5-chloro-N-( {2-oxo-3-[ 4-( 3-oxo- 4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl)-2-carboxamide, under the protection of argon at room temperature, add 46 mL of anhydrous triethylamine to (S ) -5-chloro-N-({2-oxo-3-[4-(3-oxo-4-morpholinyl)phenyl]-1,3-oxazolidin-5-yl}methyl )-2-carboxamide in 7 L of anhydrous tetrahydrofuran suspension, and then 6 mL of acetyl chloride was added dropwise, and reacted at room temperature for 2 hours. TCL (EA / MeOH=10:1) tracking shows that some raw materials still exist. Then add 25 mL of anhydrous triethylamine and 25 mL of acetyl chloride to continue the reaction for 2 hours. TCL (EA / MeOH=10 / 1)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com