A method for preparing high-purity sucrose fructan monomer by high-speed countercurrent chromatography
A high-speed countercurrent chromatography and fructotetraose technology, which is applied in the field of preparing high-purity fructan monomers, can solve the problem that high-purity fructan is difficult to prepare, is not suitable for industrialized production methods, and cannot be used as a high-purity control. To solve the problems of products, etc., to achieve the effect of solvent recycling, low production cost, and ease of separation environment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
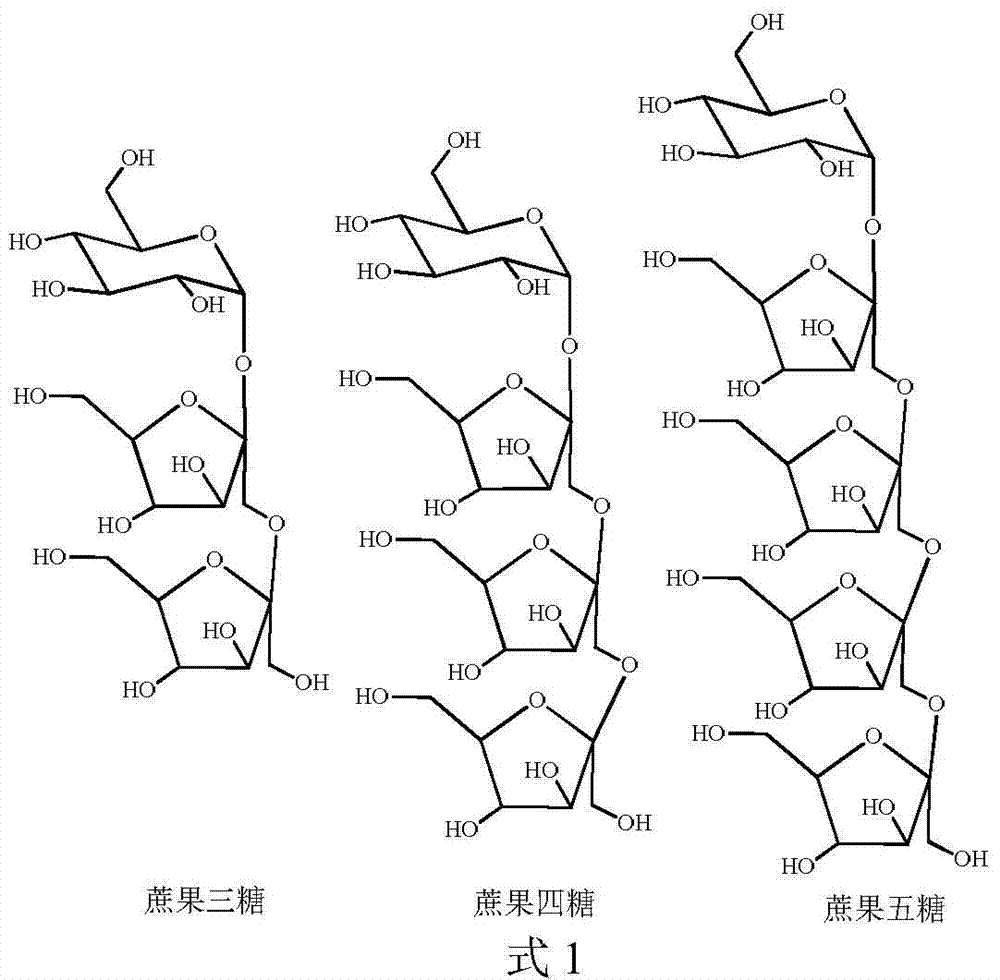
[0039] Follow the steps below to prepare high-purity fructo-oligosaccharides:
[0040] (1) Acetylation protection: Dissolve 5 g of fructo-oligosaccharide in 50 mL of pyridine, add 0.2 mol of acetic anhydride, react at room temperature for 24 hours, then concentrate under reduced pressure with a rotary evaporator to obtain yellow syrupy acetylated fructo-oligosaccharide.
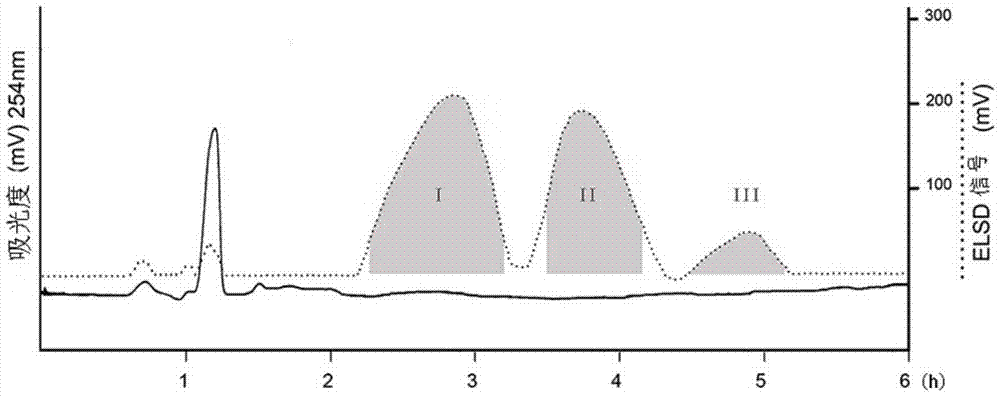
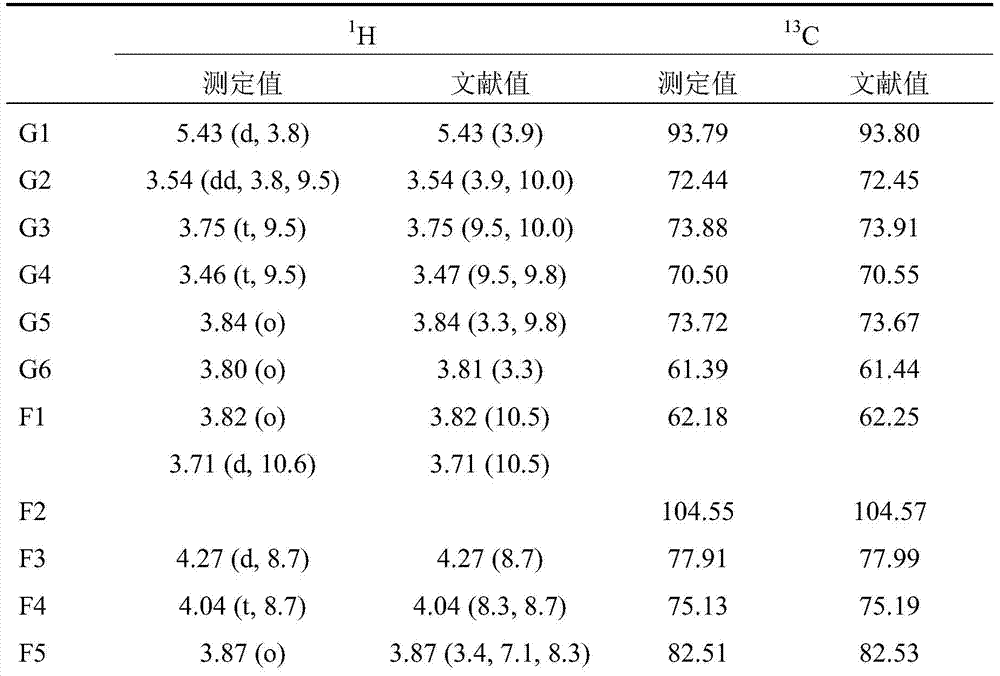
[0041](2) The solvent system of high-speed countercurrent chromatography is petroleum ether: n-butanol: methanol: water = 3:2:1:4 (volume ratio) system, and the above-mentioned solvent system is configured in a separatory funnel, shaken and left to stand Separate the upper and lower phases after equilibrating for a period of time. The upper phase is the stationary phase and the lower phase is the mobile phase. Take 150 mg of acetylated fructooligosaccharides and dissolve them in the mixture of 5 mL of the upper phase and 5 mL of the lower phase for use. The TBE-300A preparative countercurrent chromatograph pr...
Embodiment 2
[0080] (1) Methylation protection: Dissolve 5g fructooligosaccharides in 200mL 20wt% sodium hydroxide solution, add dropwise 0.5mol dimethyl sulfate under ice-water bath, stir at room temperature for 6 hours after completion, then use ethyl acetate (100mL×3) extraction, ethyl acetate partly concentrated under reduced pressure to obtain yellow syrupy methylated oligosaccharides.
[0081](2) The solvent system of high-speed countercurrent chromatography is selected from cyclohexane: n-butanol: ethanol: water = 3: 2.5: 1.5: 3.5 (volume ratio) system, the above-mentioned solvent system is configured in a separatory funnel, shaken and then static Place the layers, and after a period of equilibrium, the upper and lower phases are separated, the upper phase is the stationary phase, and the lower phase is the mobile phase, and 180 mg of methylated fructooligosaccharides is dissolved in a mixture of 5 mL of the upper phase and 5 mL of the lower phase for use. The sample was separated b...
Embodiment 3
[0087] (1) Benzylation protection: Dissolve 5 g of fructooligosaccharides in 300 mL of dimethylformamide, add 0.1 mol of sodium hydroxide in an ice-water bath, then dropwise add 0.2 mol of benzyl bromide, and then stir and react at room temperature for 3 hours. 300 mL of water was added, and then extracted with ethyl acetate (200 mL×3). The ethyl acetate part was concentrated under reduced pressure to obtain benzylated oligosaccharides in the form of yellow syrup.
[0088] (2) The solvent system of high-speed countercurrent chromatography is selected from the system of n-hexane: n-butanol: ethanol: water = 4:2:2:4 (volume ratio), and the above-mentioned solvent system is arranged in a separatory funnel, shaken up and left to stand Separate the upper and lower phases after equilibrating for a period of time. The upper phase is the stationary phase and the lower phase is the mobile phase. Take 160 mg of benzylated fructooligosaccharides and dissolve them in the mixture of 5 mL of...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
| optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com