Kit for predicting curative effect of lucentis for treating age-related macular degeneration
A macular degeneration, ranibizumab technology, used in the determination/inspection of microorganisms, DNA/RNA fragments, recombinant DNA technology, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: Blood Sample Collection and Genomic DNA Extraction
[0039]1. Cases were selected according to the revised diagnostic criteria in New York in 1984. A total of unrelated AMD patients from Beijing were selected. Among them, 55 patients with good curative effect on age-related macular degeneration treated with ranibizumab were used as the case group (age: 55-80 years old, with an average of 71 years), and 60 patients with poor efficacy in the treatment of age-related macular degeneration with ranibizumab were used as the control group (age: 56-82 years, with an average of 72 years). All subjects were of Han nationality and signed written informed consent. This study was approved by the Beijing Hospital and Beijing Institute of Gerontology Ethical Review Committee, and complied with the World Medical Association Declaration of Helsinki: Ethical Principles for Human Medical Research.
[0040] 2. According to the following method, human genomic DNA was prepared. ①...
Embodiment 2
[0041] Example 2: Identification and Identification of Variation Sites
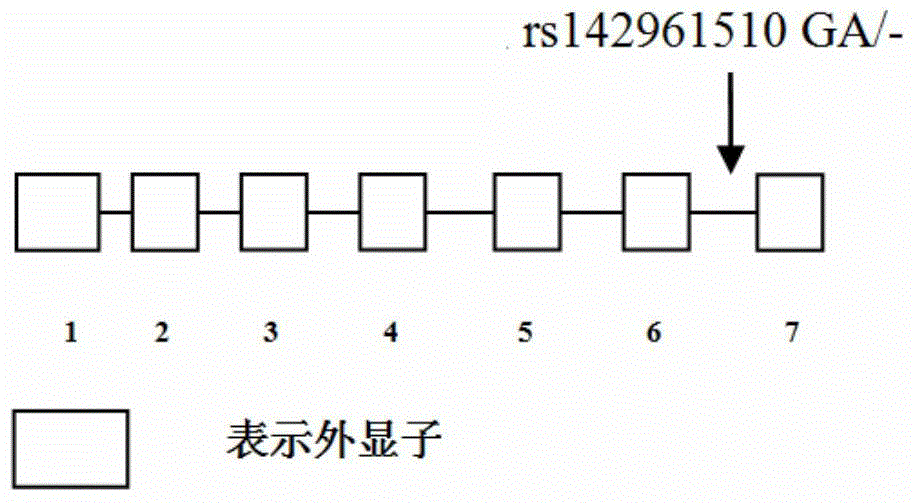
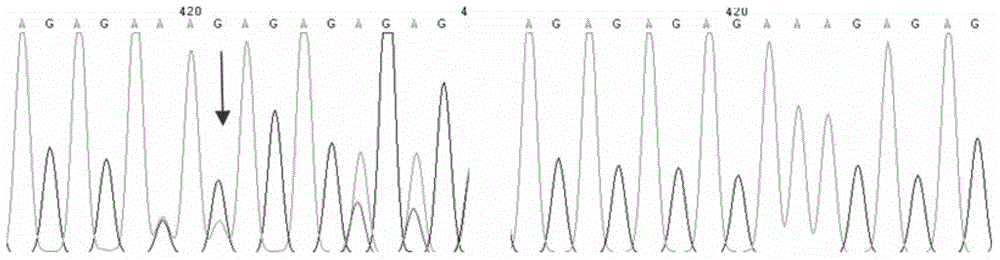
[0042] The invention adopts PCR-sequencing analysis method to detect the genotype of the +401 site (the allelic site is G / A) in the sixth intron region of the VEGF-A gene. figure 2 It is the sequencing map of the VEGF-A gene variation site.
[0043] 1. Determination of PCR-sequencing primers
[0044] The DNA base sequence (Seq ID №1) near rs142961510GA / - was retrieved from Genebank, and the primer design was completed under Oligo7.0 software. The target fragment is located in the 6th intron region of the VEGF-A gene, with a total length of 806bp. The sense strand F1 (+291bp-+310bp) and the antisense strand R1 (+473bp-+492bp) are determined. The specific primer sequences are as follows:
[0045] F1: 5'-AAAACACAGACTCGCGTTGC-3' (Seq ID NO.2)
[0046] R1: 5'-AGTTTCTAGCTGCCTGCCTG-3' (Seq ID NO.3)
[0047] 2. PCR-sequencing reaction system and conditions
[0048] Amplify a partial fragment of the 6th intr...
Embodiment 3
[0051] Example 3: Correlation between Gene SNP and AMD
[0052] Statistical methods: The Hardy-Weinberg balance test was used to study the group representativeness of the samples. The distribution frequency of alleles and genotypes of VEGF-A gene rs142961510GA / - site between the ranibizumab-treated age-related macular degeneration curative effect case group and the normal control group was calculated by Pearson chi-square test in SPSS17.0 software. The risk OR value and its 95% CI confidence interval for the efficacy of ranibizumab in the treatment of age-related macular degeneration, with P<0.05 as the standard of significant difference.
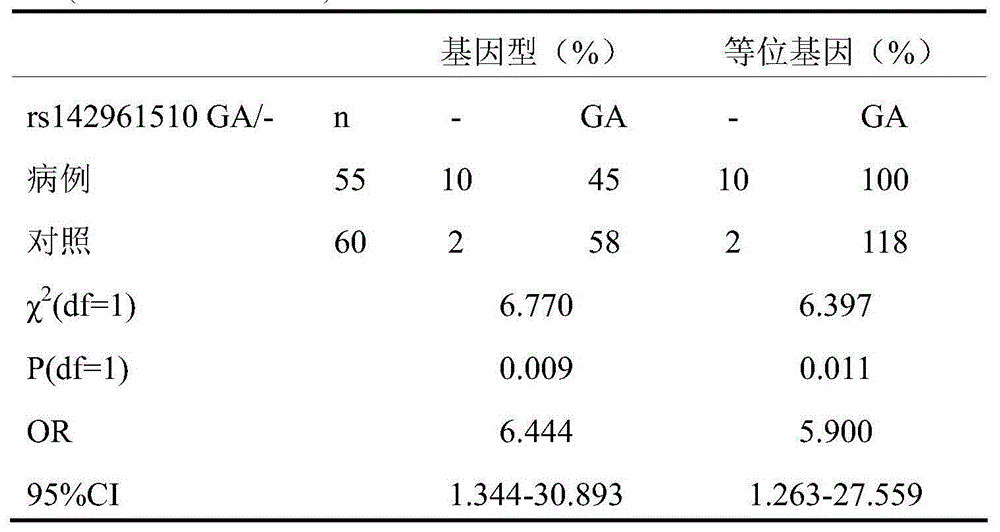
[0053] Results: The distribution of the genotype and allele frequency of the rs142961510GA / - site on the VEGF-A gene located in the 6p12 region between the cases and the control group is shown in Table 1.
[0054] Table 1 Distribution of genotype and allele frequency of VEGF-A(rs142961510GA / -) locus in case-control group
[0055]
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com