Compound PAC-1 or salt thereof, and medicinal composition containing compound or salt thereof
A technology of PAC-1 and composition, applied in the field of preparation of PAC-1), can solve the problems of strong neurotoxicity, unsatisfactory prospects, and failure to effectively control the harm.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The ZYS-1 can be prepared by, for example, the following method, but the preparation method is not limited thereto. The methods include:
[0041] Step (1): dissolving the crystal of PAC-1 in an organic solvent to obtain the first solution of PAC-1;
[0042] Step (2): under stirring, add water to the solution of PAC-1 obtained in step (1) to obtain a second solution of PAC-1;
[0043] Step (3): Stir continuously, and when the precipitated solid is white or milky white, filter, remove the solvent, and obtain the precipitate;
[0044] Step (4): drying the obtained precipitate to obtain PAC-1 in an amorphous form.
[0045] In step (1), relative to 1 g of PAC-1 crystals, the amount of organic solvent used may be, for example, 1-20 mL, preferably 2-10 mL, more preferably 4-6 mL. Ultrasonic treatment, stirring, shaking and other means can be used to promote the dissolution of PAC-1 crystals. The organic solvent may be, for example, one or more selected from absolute ethano...
Embodiment 1
[0060] Embodiment 1: Preparation of ZYS-1
[0061] Take 1 g of PAC-1 needle crystal (prepared according to the method of Quinn P. Peterson et al., J. Med. Chem. 2009, 52, 5721–5731), dissolve it in DMSO (5 mL) by ultrasonic, and stir at a speed of 500 r / min 50 mL of water was added, and the mixture was continuously stirred for 10 min until the precipitate was milky white, filtered under reduced pressure, and dried in vacuo to obtain a white solid.
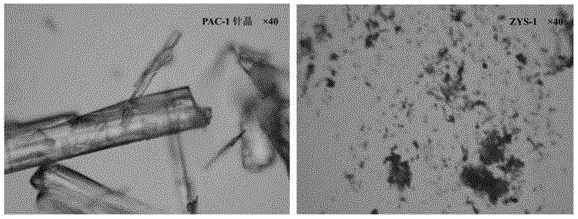
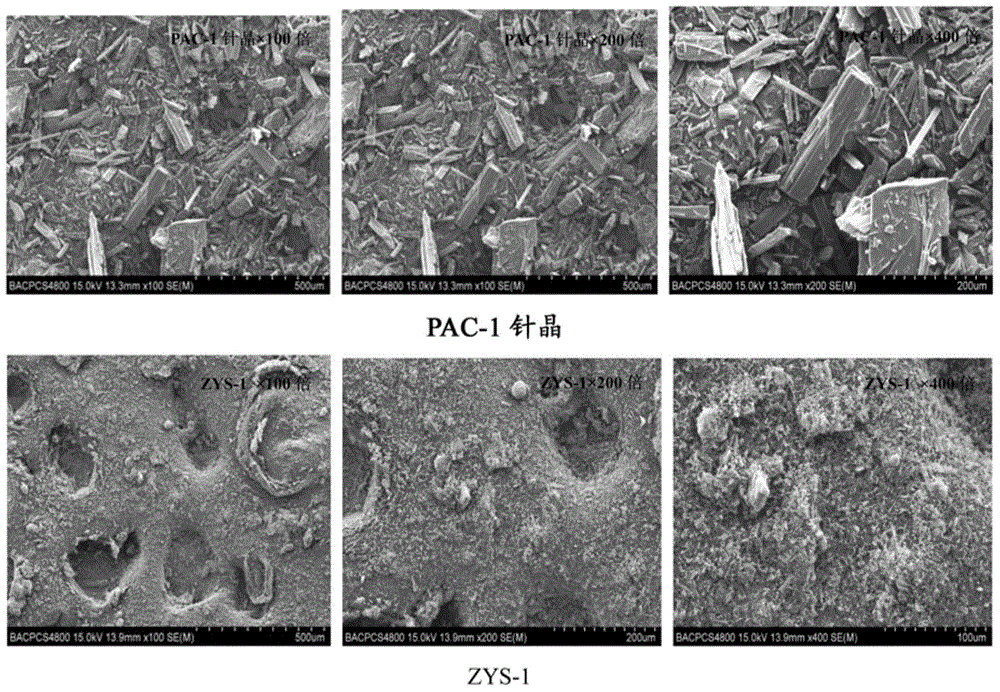
[0062] After identification by microscope, scanning electron microscope and X-powder diffraction, it is obvious that the obtained powder is amorphous PAC-1 (ZYS-1). Under the microscope and scanning electron microscope, it can be seen that the morphology of amorphous and needle crystals is completely different. The surface of needle crystals is relatively smooth, showing a typical needle crystal shape, while ZYS-1 presents a loose and porous structure. The results are shown in Figure 2-3 . Comparing the X-powder diffraction patt...
Embodiment 2
[0063] Example 2: Comparison of ZYS-1 and PAC-1 needle crystal solubility
[0064] Take an appropriate amount of ZYS-1 and PAC-1 needle crystals and dissolve them in an appropriate amount of methanol, absolute ethanol, 95% ethanol, acetonitrile, ether, ethyl acetate, propanol, and acetone, ultrasonically for 1 hour, and stand at room temperature for 24 hours. Make a supersaturated solution. Centrifuge at 4000r / min for 5min, take the supernatant and dilute appropriately, filter with a 0.45μm syringe filter, measure the concentration by HPLC, and calculate the solubility.
[0065] The experimental results show that the solubility of ZYS-1 is higher than that of PAC-1 needle crystals, and the results are shown in Table 1.
[0066] Table 1 ZYS-1 and PAC-1 needle crystal solubility comparison
[0067]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com