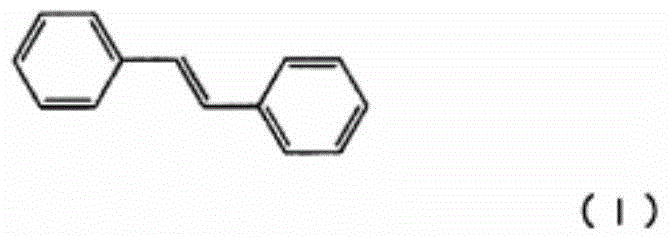
Growth hormone secretion promoter
A growth hormone and accelerator technology, applied in endocrine system diseases, pill delivery, food ingredients, etc., can solve the problems that growth hormone secretion enhancers fail to achieve the effect, and achieve a good growth hormone secretion promotion effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and comparative example 1
[0108] In Example 1, tablets (9 tablets containing 80 mg of S-adenosylmethionine agent) in which S-adenosylmethionine-containing yeast was mixed were prepared as samples. In Comparative Example 1, a placebo tablet containing no S-adenosylmethionine-containing yeast was prepared as a sample. The S-adenosylmethionine-containing yeast was "Amii" (trade name) manufactured by Iwata Chemical Co., Ltd.
[0109] The components of the sample of Comparative Example 1 and the sample of Example 1 are shown in Table 1 below.
[0110] [Table 1]
[0111] Components (one dose: content of components in 9 tablets)
Example 1
Comparative example 1
The content of S-adenosylmethionine in yeast containing S-adenosylmethionine (mg)
80.0
0.0
Weight of S-adenosylmethionine-containing yeast (dry yeast) (mg)
1530.0
0.0
Excipient (crystalline cellulose) (mg)
270.0
1800.0
[0112] (Evaluation of Growth Hormone Secretion-Stimulating Effe...
Embodiment 2 and comparative example 2
[0124] Except having used the sample whose composition is shown in Table 2, the same test and evaluation as Example 1 were performed.
[0125] [Table 2]
[0126] Components (one dose: content of components in 9 tablets)
Example 2
Comparative example 2
The content of S-adenosylmethionine in yeast containing S-adenosylmethionine (mg)
40.0
0.0
Weight of S-adenosylmethionine-containing yeast (dry yeast) (mg)
765.0
0.0
Excipient (crystalline cellulose) (mg)
1035.0
1800.0
[0127] The rate of change in the amount of GH in the urine of each member of the group was calculated by formula (1), and the average value of the rate of change in the amount of GH in the urine of 13 people was 112.4%.
[0128] The results of Examples 1 and 2, and Comparative Examples 1 and 2 show that GH secretion of a living body can be effectively promoted by administering S-adenosylmethionine and / or its salt to the living body.
[0129] Th...
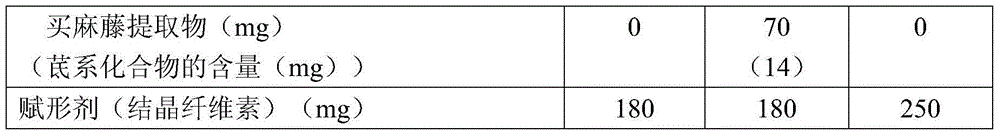
Embodiment 3、 Embodiment 4 and comparative example 3
[0141] Tablets mixed with grape extract were prepared as samples of Example 3. As a sample of Example 4, a tablet mixed with the extract of Matonia japonica was prepared. As a sample of Comparative Example 3, tablets containing placebos containing no grape extract and safflower extract were prepared. As the grape extract, VINEATROL 20M (trade name) manufactured by ACTICHEM was used. As the sage extract, sage resveratrol-20 (trade name) produced by YBF Co., Ltd. was used.
[0142] Each composition of the example sample and the comparative example sample of the growth hormone secretion promoter concerning this invention is shown in detail in Table 3 below.
[0143] [table 3]
[0144]
[0145]
[0146] (Effect Evaluation of Growth Hormone Secretion Promoter)
[0147] Let six adult men and women in the group ingest the sample of the above-mentioned Example 3 one hour before going to bed for 4 consecutive days, and measure the content of growth hormone in the initial urin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com