Diagnostic markers for treating cell proliferative disorders with telomerase inhibitors
A telomerase inhibitor, cancer cell technology, applied in the treatment of these individuals, can solve the problem of cancer patients not getting benefits, exposure to toxic effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0168] Embodiment 1: Oligonucleotide N3'→P5'phosphoramidate (NP) or N3'→P5'thiophosphoramidate (NPS) preparation and lipid conjugation
[0169] This example shows how to synthesize lipid-conjugated oligonucleotides N3'→P5' phosphoramidate (NP) or N3'→P5' phosphorothioate (NPS).
[0170] Materials and methods
[0171] starting compound
[0172] These compounds can be prepared as described, for example, in McCurdy et al., Tetrahedron Letters 38:207-210 (1997) or Pongracz & Gryaznov, Tetrahedron Letters 49:7661-7664 (1999). The starting 3'- Amino nucleoside monomers.
[0173] lipid attachment
[0174] Depending on the nature of the linkage chosen, a variety of synthetic methods can be used to conjugate the lipid moiety L to oligonucleotides; see, e.g., Mishra et al., Biochim. et Biophys. Acta 1264:229-237 (1995), Shea et al. al., Nucleic Acids Res. 18:3777-3783 (1995), or Rump et al., Bioconj. Chem. 9:341-349 (1995). Typically, conjugation is achieved through the use of...
Embodiment 2
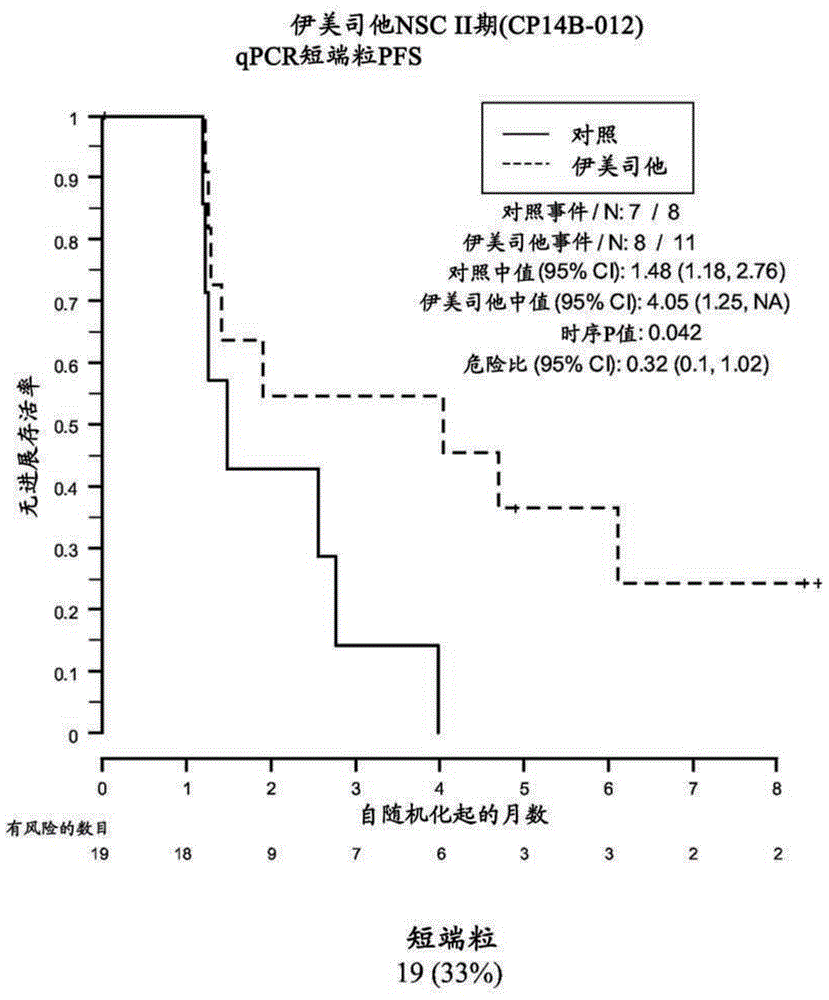
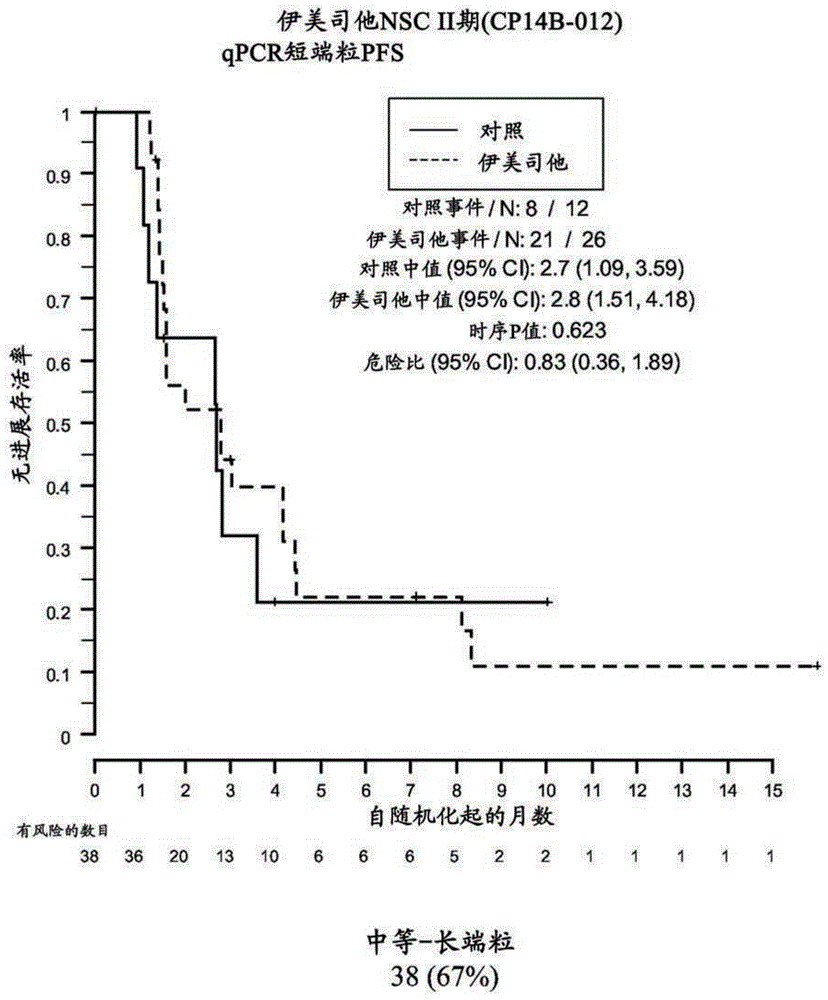
[0183] Example 2: Analysis of formalin-fixed, paraffin qPCR of embedded samples
[0184] This example demonstrates the performance of quantitative polymerase chain reaction for the determination of relative telomere length of FFPE NSC Phase II (CP14B-012) research tissue samples.
[0185] Materials and methods
[0186] clinical trial design
[0187] The purpose of the NSC Phase II (CP14B-012) study was to evaluate the efficacy and safety of imetelostat (GRN163L) as maintenance therapy in patients with advanced-stage NSCLC who have not progressed after 4 cycles of platinum-based therapy . Participants were randomized in a 2:1 ratio to imelastat and standard of care versus standard of care alone. Participants who received bevacizumab along with their induction chemotherapy continued to receive bevacizumab in this study.
[0188] The primary outcome measure was progression-free survival, defined as the time from randomization to documented disease progression or death, wh...
Embodiment 3
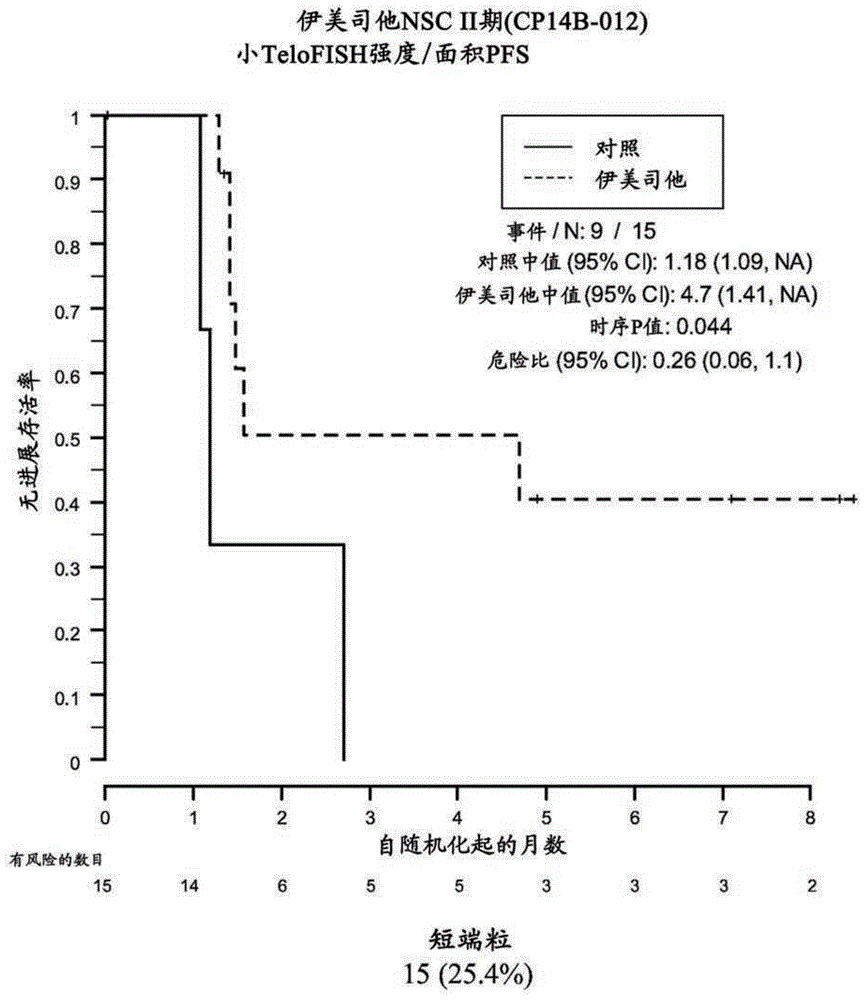
[0211] Example 3: Analysis of formalin-fixed, paraffin-embedded samples from the NSC Phase II (CP14B) study Telo-FISH
[0212] Samples were obtained from 61 of 116 patients enrolled in the NSC Phase II (CP14B-012) study described above. Of these 61 patient samples, 59 yielded evaluable Telo-FISH assay results for PFS analysis. Each assay yielded data from 7-14545 foci in 6 regions ("fields") on the slide. Area and fluorescence intensity were recorded for each focus.
[0213] Materials and methods
[0214] Unstained FFPE tissue slides (5 μm thick tissue sections) were prepared by conventional histological methods. Tissue slides were preheated at 65°C for 6 minutes to melt the paraffin before loading onto slide holders. Immerse the loaded slide rack in 100 mL of xylene in the staining jar for 2 times for 3 minutes (3 minutes x 2) to remove paraffin.
[0215] Slides were then hydrated in 3 minute increments via a graded ethanol series: 100% EtOH (3 minutes x2), 95% EtOH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com