Electrochemical sensor based on double metal porphyrin coordination polymer and its preparation method
A technology of coordination polymers and double metal porphyrins, applied in the direction of material electrochemical variables, can solve the problems of electrochemical sensors that have not been reported yet, achieve good electrocatalytic performance, fast detection speed, and improve electrical sensing performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
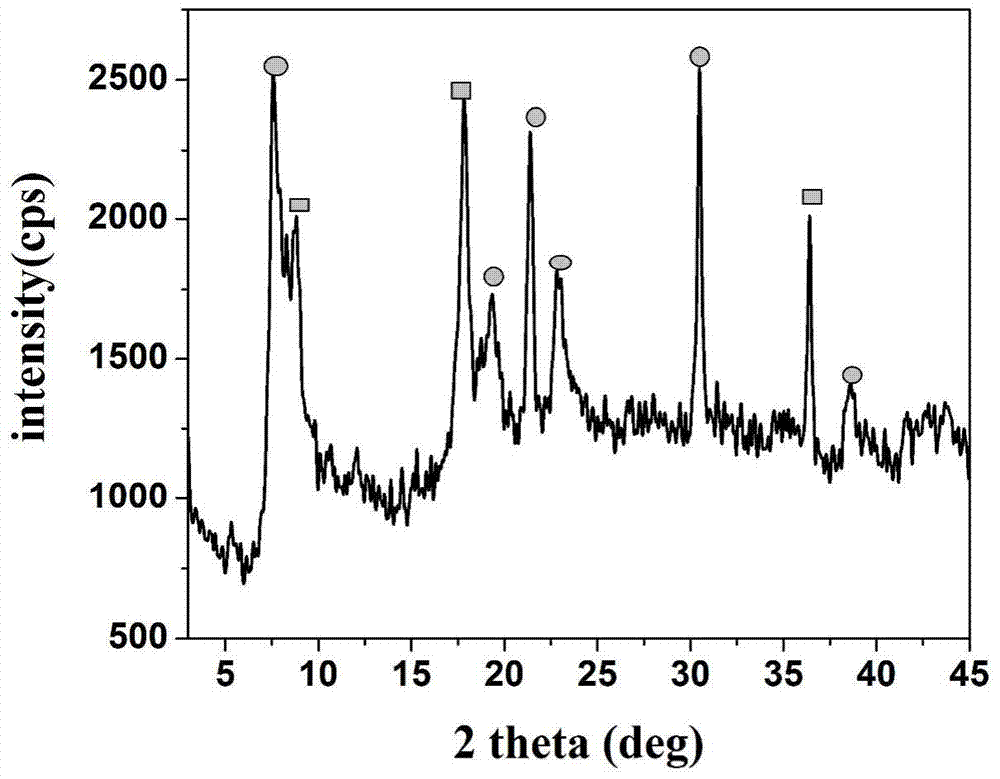
[0041] Example 1 Bimetallic coordination polymer [Cu 2 (Co-TCPP)(H 2 O) 2 ]·0.5DMF·5H 2 Preparation of O(CoTCPP-Cu)
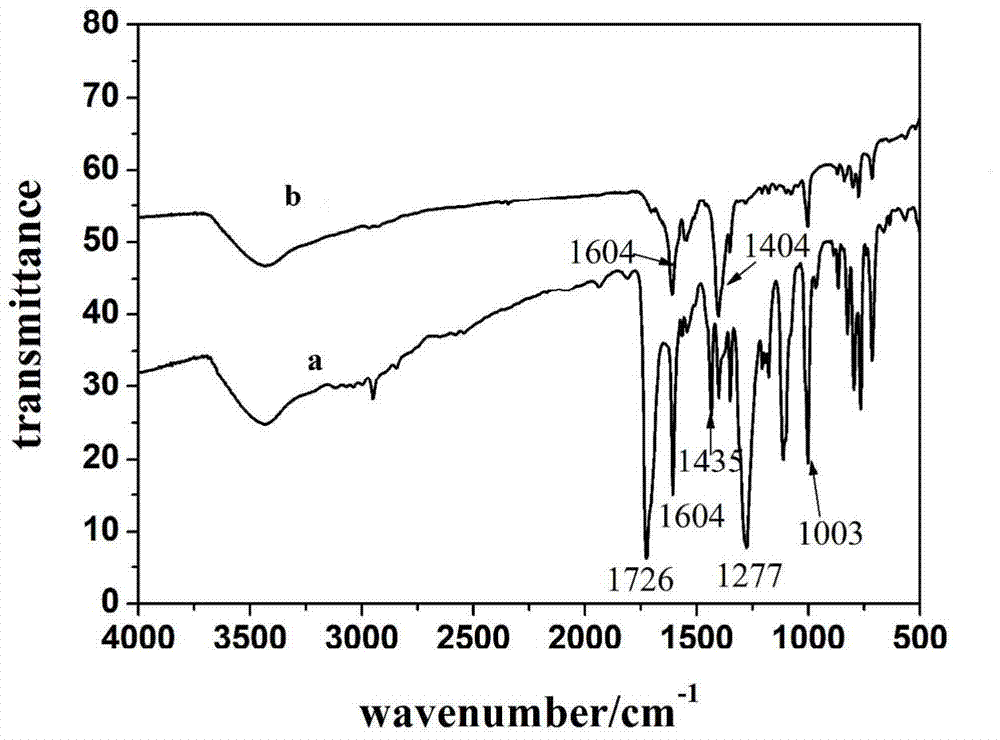
[0042] Weigh CoTCPP 3mg (0.01mmol), add DMF 3mL to dissolve it; meanwhile weigh excess Cu(NO 3 ) 2 .3H 2 O 100mg (0.4mmol), add DMF 2mL to dissolve it. The prepared Cu(NO 3 ) 2 The solution was added to the CoTCPP solution, and HNO was added while stirring 3 (1M) 1-4 mL, and finally a mixed solution with red flocs precipitated was obtained. The mixed solution is heated to carry out solvothermal reaction, and left to stand in an oven at 65-100° C. for 5 days to obtain a purple-red powder. Filtration, respectively with DMF, H 2 O and EtOH washed, and dried at room temperature.
[0043] The synthesis of CoTCPP can refer to literature: (a) Lindsey, J.S., H.C.Hsu, and I.C.Schreiman, SYNTHESIS OF TETRAPHENYLPORPHYRINS UNDER VERYMILD CONDITIONS. Tetrahedron Letters, 1986.27 (41): 4969-4970. (b) Kumar, A., et al. , One-pot general synthesis of metalloporph...
Embodiment 2
[0045] Example 2 Preparation of Electrochemical Sensor-I
[0046] (1) Polishing and cleaning of bare glassy carbon electrodes
[0047] Wash the glassy carbon electrode with secondary deionized water and ultrasonically for one minute, then polish it with alumina powder with a diameter of 0.3um for five minutes, wash the grinding cloth and the slurry on the electrode with secondary deionized water, and Put the glassy carbon electrode in the secondary deionized water for one minute, after repeated grinding and cleaning, finally dry the glassy carbon electrode for later use.
[0048] (2) Electrode modification
[0049] Ultrasonic dispersion of 5 mg of CoTCPP-Cu in 400 μL deionized water to form a suspension, 6 μL of the suspension was drop-coated on the surface of the glassy carbon electrode obtained in step (1), and dried; then drop-coated 2 μL of 1% nafion solution on the electrode surface , and dried to obtain the CoTCPP-Cu modified electrode, which is denoted as electric sen...
Embodiment 3
[0050] Embodiment 3 Electrochemical sensor-I is used for the detection of hydrogen peroxide
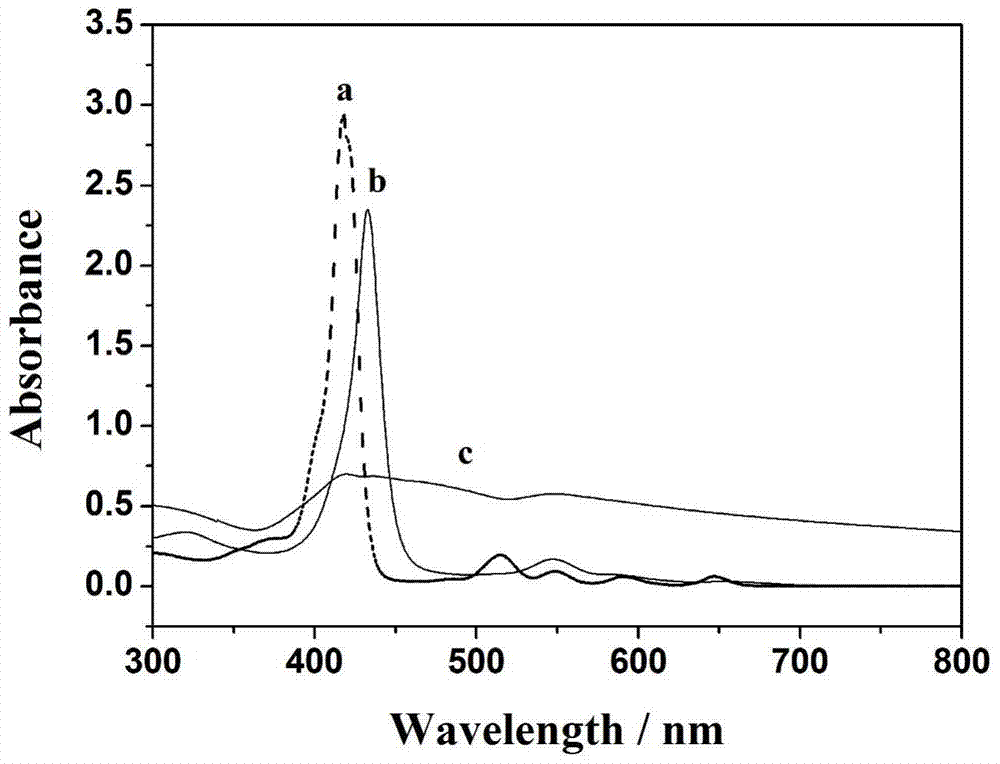
[0051] Measure the C-V curves of the bare electrode and the electrochemical sensor-I with or without hydrogen peroxide in the PBS electrolyte solution of pH=7, as shown in the attached Figure 5A Shown, a is naked GCE, b is naked GCE plus 0.5mmolL -1 h 2 o 2 , the comparison between a and b shows that the bare GCE has an effect on H 2 o 2 No response. c-d are electrochemical sensor-I adding different concentrations of H 2 o 2 0 and 0.5 mmol L -1 , with the addition of hydrogen peroxide, the response of the reduction current gradually increased, indicating that the electrochemical sensor-I had an electrocatalytic effect on the reduction of hydrogen peroxide.
[0052] The electrochemical detection of hydrogen peroxide was carried out by electrochemical sensor-I, with Figure 6A As shown, at the detection potential of -0.25V, the current-time curves of the electrochemical sensor...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com