Bcr-Abl amphiploid inhibitor and preparation method and application thereof
A technology of usage and molar volume, applied in the field of Bcr-Abl diploid inhibitors, can solve the problems of decreased affinity, drug resistance, decreased affinity between imatinib and Abl kinase, etc., and achieves a preparation method. Simple, low cost, good application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
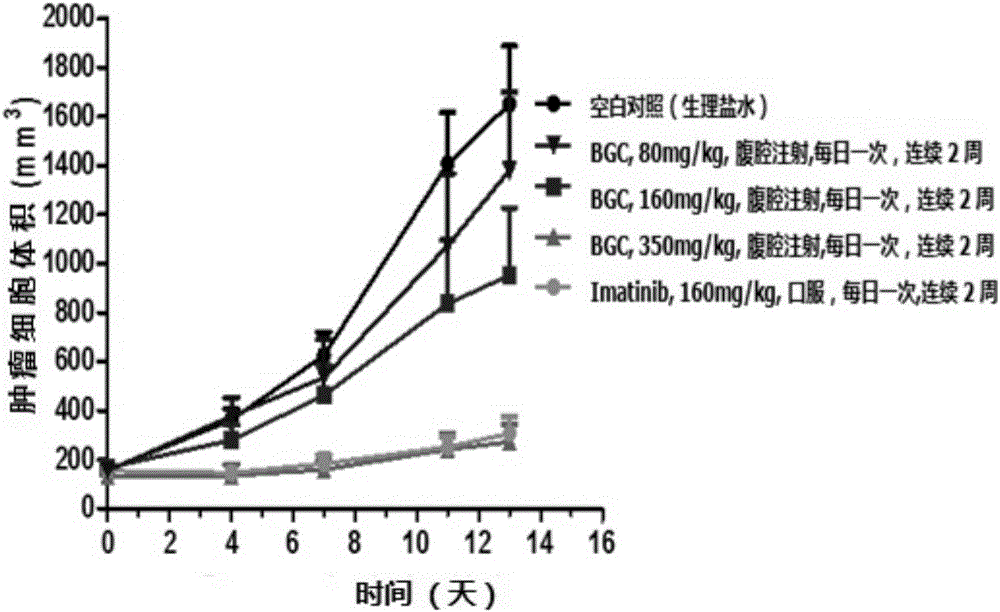
Image
Examples
Embodiment 1
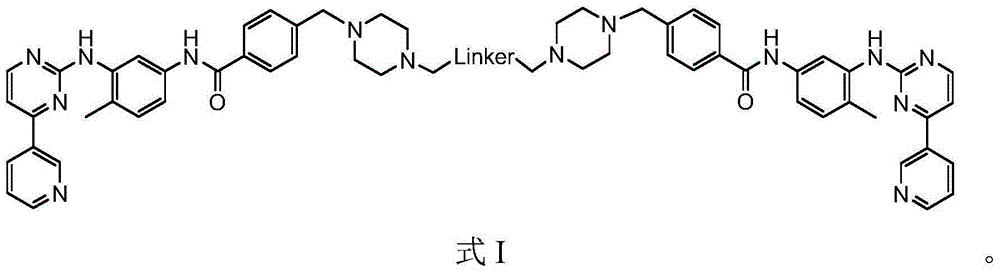
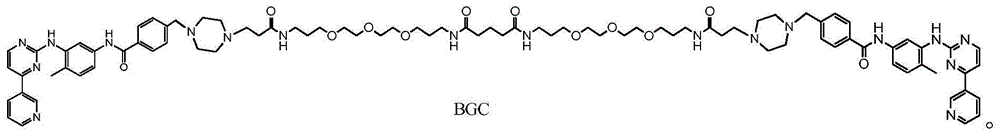
[0052] Embodiment 1 The preparation of compound BGC of the present invention
[0053] The synthetic route is as follows:
[0054]
[0055] (2) Preparation of compound BGC:
[0056]
[0057] Concrete synthetic steps are as follows:
[0058] a) preparation of compound 2
[0059] Compound 1 (5.0g, 18.0mmol; manufacturer: Shanghai Xugang Biotechnology Co., Ltd.), triethylamine (3.6g, 35.6mmol) were dissolved in dichloromethane (400ml), and 4-(chloromethyl) Benzoyl chloride (4.0g, 21.3mmol) was dissolved in dichloromethane (100ml), and was added dropwise to the reaction solution at 0°C. After the addition, it was naturally raised to room temperature and stirred for 18h. A solid precipitated in the reaction bottle. The reaction mixture was filtered, and the collected solid was washed with dichloromethane and water to obtain compound 2 as a yellow solid (5.3 g; yield 68%).
[0060] 1 H NMR (400MHz, DMSO-d6): δ10.23(s, 1H), 9.28(s, 1H), 8.97(s, 1H), 8.69(d, J=4.4Hz, 1H), 8....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com