Device for bleaching nitric acid solution through urea method and preparation method therefor
A technology of nitric acid solution and urea, applied in chemical instruments and methods, dissolving, mixing machines, etc., can solve the problems of consuming large electric energy or heat energy, not being able to solve dilute nitric acid, and taking a long time, so as to reduce the amount of addition, respond quickly, The effect of preventing environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
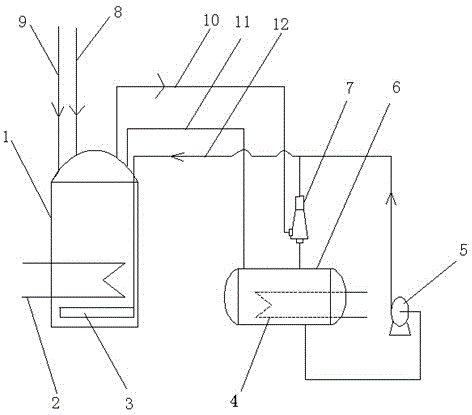
[0032] Such as figure 1 As shown, a device for bleaching nitric acid solution by the urea method comprises an acid distribution tank 1 and a urea storage tank 6, and the acid distribution tank 1 is connected with the urea storage tank 6 through a communication conduit 11; the top of the acid distribution tank 1 passes through The nitrogen dioxide pipe 10 is connected to the inlet end of the injector 7, and the other inlet end of the injector 7 is connected to the urea storage tank 6, and the outlet end of the injector 7 is horizontally arranged on the acid distribution tank through the urea pipe 12. 1 is connected to the internal liquid distributor 3; the outlet of the urea storage tank 6 is respectively connected to the urea pipe 12 and the injector 7 through the circulation delivery pump 5.
[0033] Further, the acid distribution tank 1 is provided with a first cooling pipe 2 , and the urea storage tank 6 is provided with a second cooling pipe 4 .
[0034] A concentrated ac...
Embodiment 2
[0038] A kind of preparation method of urea method bleaching nitric acid solution, comprises the following steps:
[0039] (1) Turn on the circulating delivery pump, and circulate the urea solution in the urea storage tank through the injector, so that the inside of the urea storage tank and the acid distribution tank connected with the urea storage tank are all pumped into a slight negative pressure of -10Pa;
[0040] (2) Turn off the circulating delivery pump, feed dilute nitric acid and concentrated nitric acid into the acid distribution tank at the same time, and make nitric acid of required concentration;
[0041] (3) NO produced in the acid distribution tank 2 It escapes from its top outlet and is introduced into the injector through the nitrogen dioxide pipe to mix with the urea solution entering the injector;
[0042] (4) Add urea solution with a concentration of 32.5% of 1 / 10,000 of the total mass of nitric acid in the acid distribution tank to the acid distribution ...
Embodiment 3
[0044]A kind of preparation method of urea method bleaching nitric acid solution, comprises the following steps:
[0045] (1) Turn on the circulating delivery pump, and circulate the urea solution in the urea storage tank through the injector, so that the interior of the urea storage tank and the acid distribution tank connected with the urea storage tank are all pumped into a slight negative pressure of -5Pa;
[0046] (2) Turn off the circulating delivery pump, feed dilute nitric acid and concentrated nitric acid into the acid distribution tank at the same time, and make nitric acid of required concentration;
[0047] (3) NO produced in the acid distribution tank 2 It escapes from its top outlet and is introduced into the injector through the nitrogen dioxide pipe to mix with the urea solution entering the injector;
[0048] (4) Add urea solution with a concentration of 32.5% of 1.5% of the total mass of nitric acid in the acid distribution tank to the acid distribution tank...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com