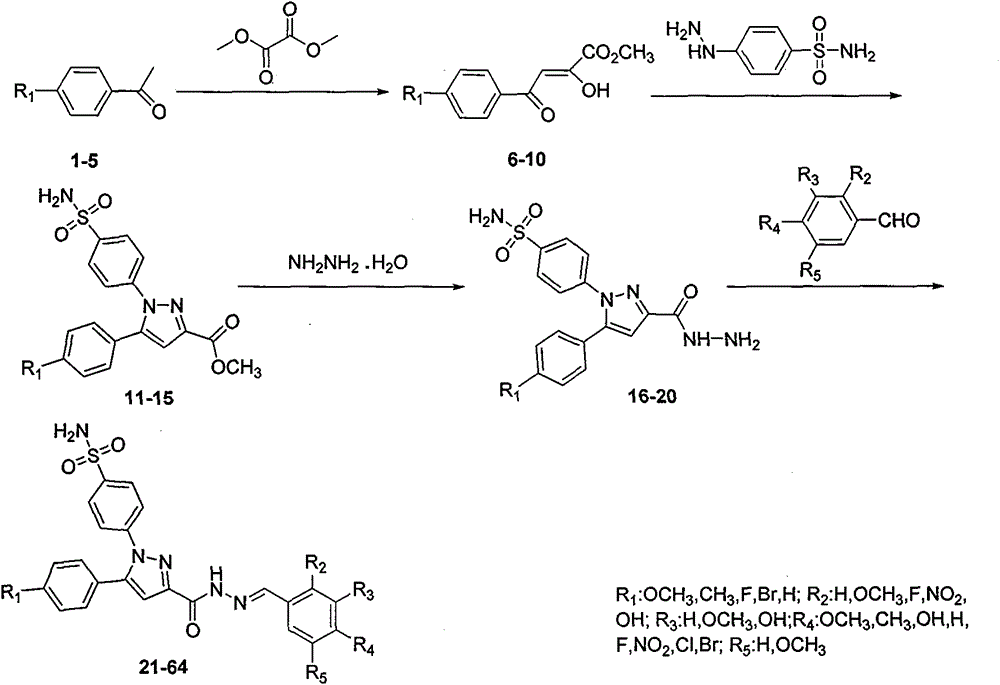
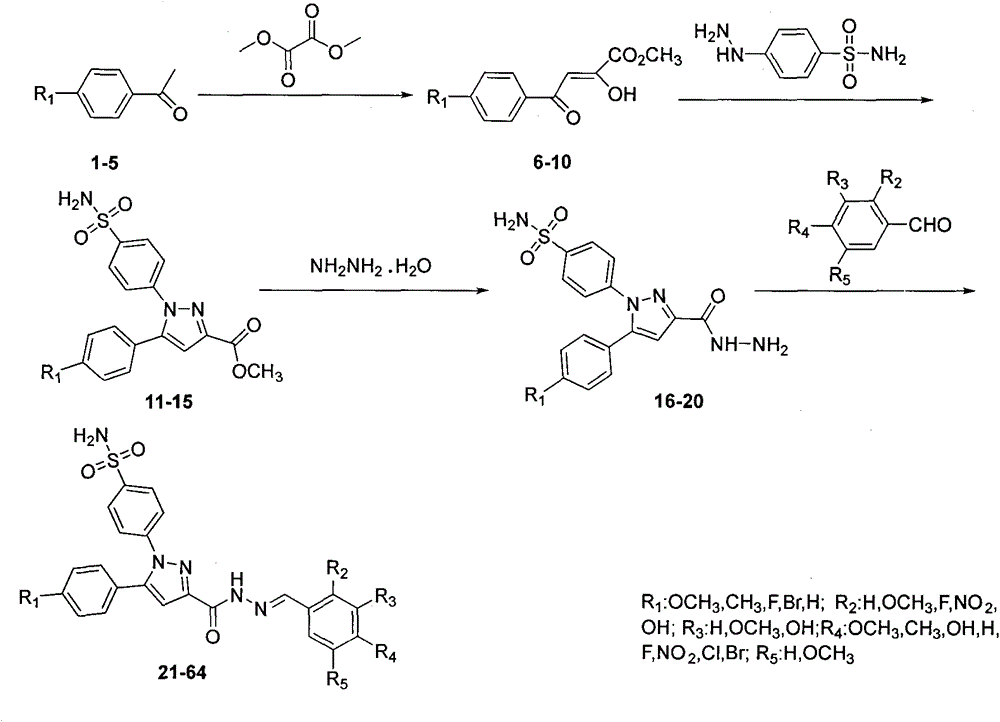
Synthesis for sulfaphenazole acylhydrazone derivatives and application of sulfaphenazole acylhydrazone derivatives in anti-cancer drugs
A technology of sulfaphenylpyrazole acylhydrazone and sulfaphenizone acylhydrazone, which is applied in the synthesis of a class of sulfaphenizone pyrazole acylhydrazone derivatives and the application field in anticancer drugs, and can solve the limitation of application range and the use of Reduced range, no bactericidal effect, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
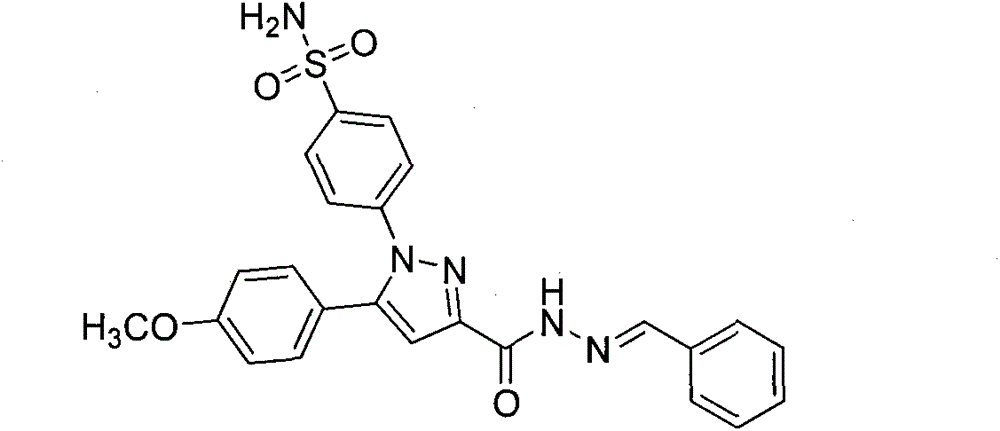
[0015] Example 1: Preparation of 4-(3-(benzoylhydrazone)-5-(4-methoxyphenyl)-1H-pyrazole)benzenesulfonamide
[0016]
[0017] Under stirring, 5-(4-methoxyphenyl)pyrazole hydrazide benzenesulfonamide (0.1g, 0.25mmol), ethanol (10mL), benzaldehyde (0.039g, 0.375mmol), acetic acid (0.5mL) Add to a 50mL round-bottom flask, react at room temperature for 12h, TLC follows the reaction (developer V AcOEt : V PE =1:2), after the reaction, filter, wash the solid with cold ethanol and distilled water in turn, and finally vacuum dry. The obtained solid is dissolved in absolute ethanol and recrystallized and purified to obtain the powdery target compound.
[0018] A white solid was obtained with a yield of 60%.m.p.251~253℃; 1 HNMR(DMSO-d 6 , 300MHz) δ: 11.81 (s, 1H, CONH), 8.55 (s, 1H, CHN), 7.90 (d, J = 6.3 Hz, 2H, ArH), 7.72 (d, J = 5.1 Hz, 2H, ArH) , 7.60(d, J=6.3Hz, 2H, ArH), 7.48(t, J=6.3Hz, 5H, ArHandSO 2 NH 2 ), 7.27(d, J=6.5Hz, 2H, ArH), 7.12(s, 1H, CH), 6.98(d, J=6.4Hz, 2H, ArH), 3.7...
Embodiment 2
[0019] Example 2: Preparation of 4-(3-(4-methoxybenzoylhydrazone)-5-(4-methoxyphenyl)-1H-pyrazole)benzenesulfonamide
[0020]
[0021] The preparation method is the same as in Example 1. White solid, yield 41%, m.p.169~171℃; 1 HNMR(DMSO-d 6 , 300MHz) δ: 11.66 (s, 1H, CONH), 8.48 (s, 1H, CHN), 7.89 (t, J=6.4Hz, 2H, ArH), 7.66 (d, J=6.5Hz, 2H, ArH) , 7.59(d, J=6.4Hz, 2H, ArH), 7.49(s, 2H, SO 2 NH 2 ), 7.26 (d, J = 6.5 Hz, 2H, ArH), 7.10 (s, 1H, CH), 7.03 (d, J = 6.5 Hz, 2H, ArH), 6.98 (d, J = 6.5 Hz, 2H, ArH), 3.82(s, 3H, OCH 3 ), 3.78(s, 3H, OCH 3 ).ESI-MS: 506.1[M+H] + .Anal.CalcdforC 25 H 23 N 5 O 5 S: C, H, N.
Embodiment 3
[0022] Example 3: Preparation of 4-(3-(2-methoxybenzoylhydrazone)-5-(4-methoxyphenyl)-1H-pyrazole)benzenesulfonamide
[0023]
[0024] The preparation method is the same as in Example 1. A white solid was obtained with a yield of 58.6%. m.p. 157~160℃; 1 HNMR(DMSO-d 6 , 300MHz) δ: 11.86 (s, 1H, CONH), 8.89 (s, 1H, CHN), 7.89 (t, J=6.4Hz, 3H, ArH), 7.60 (d, J=6.4Hz, 2H, ArH) , 7.50(s, 2H, SO 2 NH 2 ), 7.43 (t, J = 6.4 Hz, 1H, ArH), 7.27 (d, J = 6.5 Hz, 2H, ArH), 7.12 (d, 2H, CHandArH), 7.04 (t, J = 5.6 Hz, 1H, ArH), 6.99(d, J=6.5Hz, 2H, ArH), 3.87(s, 3H, OCH 3 ), 3.79(s, 3H, OCH 3 ).ESI-MS: 506.1[M+H] + .Anal.CalcdforC 25 H 23 N 5 O 5 S: C, H, N.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com