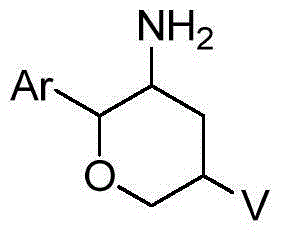
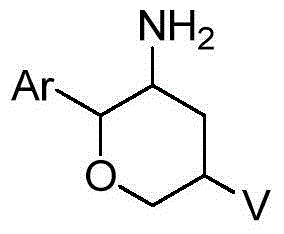
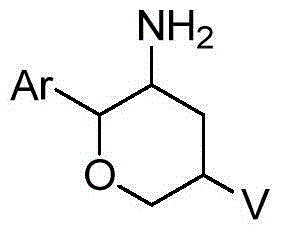
Amino six-membered ring derivative and application thereof in medicines
A technology of amino six-membered rings and derivatives, which can be used in pharmaceutical formulations, medical preparations containing active ingredients, drug combinations, etc., and can solve problems such as the burden of diabetes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0333] (S)-Ethyl 5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)-tetrahydro-2H-pyran-3-yl)-1-( Methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate (Compound 1)
[0334] (S)-ethyl5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)-tetrahydro-2H-pyran-3-yl)-1-(methylsulfonyl)-1,4, 5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate
[0335]
[0336]
[0337] The first step: (S)-ethyl 1-(methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate (1a)
[0338] (S)-ethyl1-(methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate
[0339] Dissolve Intermediate 2 (0.3g, 0.84mmol) in dichloromethane (1mL) at room temperature, cool down to 0°C, add trifluoroacetic acid (3mL), stir and react at 0°C for 2 hours, then concentrate the reaction solution under reduced pressure . Purified by column chromatography [(dichloromethane / methanol (v / v)=40:1-20:1), adding a small amount of ammonia water] to obtain off-white solid 1a (0.22 g, yield 100%).
...
Embodiment 2
[0351] (S)-5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)-tetrahydro-2H-pyran-3-yl)-1-(methyl Sulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazol-4-yl)methanol (Compound 2)
[0352] ((S)-5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)-tetrahydro-2H-pyran-3-yl)-1-(methylsulfonyl)-1,4 ,5,6-tetrahydropyrrolo[3,4-c]pyrazol-4-yl)methanol
[0353]
[0354] The first step: (S)-tert-butyl 4-(hydroxymethyl)1-(methylsulfonyl)pyrrolo[3,4-c]pyrazole-4,5-(1H,4H,6H)- Carboxylate (2a)
[0355] (S)-tert-butyl4-(hydroxymethyl)-1-(methylsulfonyl)pyrrolo[3,4-c]pyrazole-5(1H,4H,6H)-carboxylate
[0356] Intermediate 2 (1.4g, 3.9mmol) was dissolved in tetrahydrofuran (15mL) at room temperature, cooled to 0°C, lithium borohydride (0.206g, 9.48mmol) was added, and allowed to rise to room temperature for 5 hours. The reaction solution was cooled to 0°C, saturated ammonium chloride solution (20 mL) was added, extracted with ethyl acetate (30 mL×3), the organic phases were combined, and washed with satu...
Embodiment 3
[0372] (R)-Methyl 5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl)-1-(methyl Sulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate (Compound 3)
[0373] (R)-methyl5-((3R,5S,6R)-5-amino-6-(2,5-difluorophenyl)tetrahydro-2H-pyran-3-yl)-1-(methylsulfonyl)-1,4,5 ,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate
[0374]
[0375] The first step: (R)-methyl 1-(methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate (3a)
[0376] (R)-methyl1-(methylsulfonyl)-1,4,5,6-tetrahydropyrrolo[3,4-c]pyrazole-4-carboxylate
[0377] Intermediate 3 (1 g, 2.90 mmol) was dissolved in dichloromethane (15 mL) at room temperature, cooled to 0 °C, trifluoroacetic acid (5 mL) was added, and reacted at 0 °C for 7.5 hours. Concentrate the reaction solution under reduced pressure, add dichloromethane (20mL), methanol (1mL) and 5 drops of ammonia water to mix the sample, and purify by column chromatography [(dichloromethane / methanol (v / v)=40:1-20:1 ), add...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com