Application of carbonyl reductase gene, engineering bacteria containing the gene and method for catalyzing reduction reaction
A catalyst and reaction system technology, applied in the asymmetric reduction of carbonyl compounds, carbonyl reductase and its gene field, can solve the problems of environmental pollution, high reaction energy consumption, and difficulty in catalyst recovery, so as to increase the reaction concentration and reduce the Toxicity, efficiency-enhancing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the acquisition of YlCR2 gene
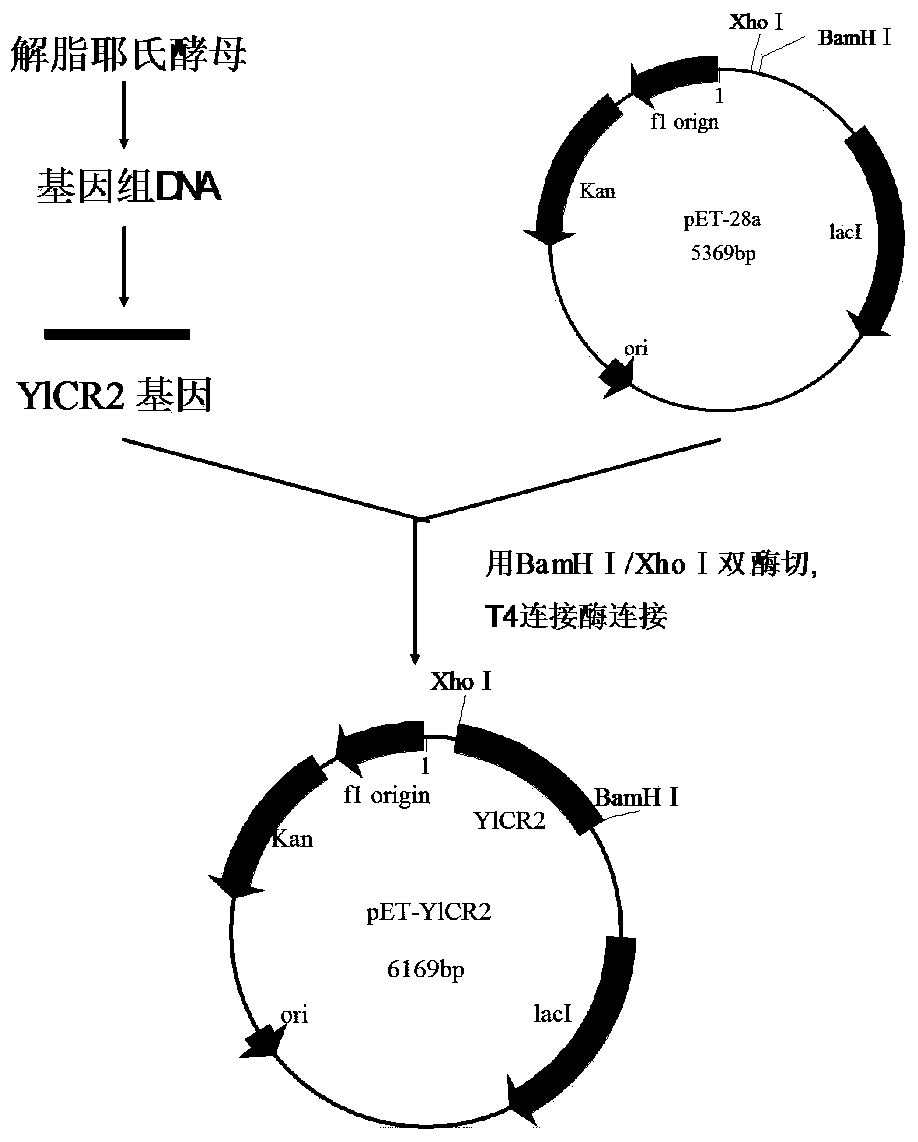
[0047] figure 1 It is the construction diagram of the carbonyl reductase gene YlCR2. According to the gene sequence (Genbank accession number: ) that is predicted to be Yarrowia lipolytica (yarrowia liplytic) reductase included in Genbank, design PCR primers as follows:
[0048] Upstream primer: CG GGATCC ATGCCTGCACCAGCAAC
[0049] Downstream primer: CCG CTCGAG TCAAGGACAACAGTAGCCGCGC
[0050] Wherein, the underlined part of the upstream primer is the BamHI restriction site, and the underlined part of the downstream primer is the XhoI restriction site.
[0051] PCR amplification was performed using the genomic DNA of Yarrowia lipolytica as a template. PCR system (total volume 50μL) is: ddH 2 O 19 μL, 2×Taq Plus Master Mix (Dye Plus) 25 μL, genomic DNA 2 μL, upstream primer and downstream primer 2 μL each. The PCR reaction conditions were: 94°C for 5 min; 30 cycles of 94°C for 30 s, 56°C for 30 s, 72°C for 1 min; 72°...
Embodiment 2
[0052] Embodiment 2: the acquisition of recombinant bacterial strain E.coli BL21 / pETY1CR2
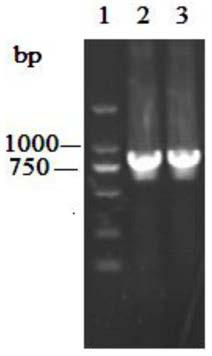
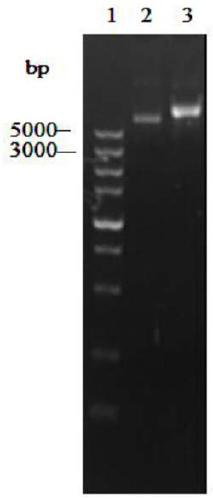
[0053] The recombinant plasmid pMD19-T-YlCR2 and the commercial vector pET28a were digested with restriction endonucleases BamHI and XhoI respectively. After the treatment, the DNA fragments were ligated by sticky ends to obtain the recombinant expression vector pET28a-YlCR2. Transform the recombinant expression vector pET28a-YlCR2 into Escherichia coli BL21(DE3), smear it on an LB plate containing kanamycin resistance at a final concentration of 50ug / mL, and randomly pick positive clones. For the recombinant plasmid, see image 3 , colony PCR see Figure 4 , Sequencing verification, using software to analyze the sequencing results, the sequence is identical to the nucleotide sequence of the YlCR2 gene. The result shows that recombinant E. coli E.coli BL21 / pETYlCR2 has been obtained
Embodiment 3
[0054] Embodiment 3: the cultivation of recombinant bacteria
[0055] Inoculate the recombinant Escherichia coli E.coli BL21 / pETY1CR2 thallus obtained in Example 2 into 50 mL of LB liquid medium containing 50 ug / mL kanamycin, and cultivate overnight at 37° C. with shaking at 200 rpm; Inoculated in 50 mL of LB liquid medium containing 50 ug / mL kanamycin, cultured at 37°C with shaking at 200 rpm to OD600 0.8, added inducer isopropyl-β-D-thiogalactoside ( IPTG) 0.1 mmol / L, induced culture at 30° C. for 10 h; centrifuge at 5000 rpm for 10 min, collect the bacterial cells, wash with physiological saline, and collect whole cells of the recombinant bacteria. The bacterium can be used directly as a biocatalyst or for protein purification.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com