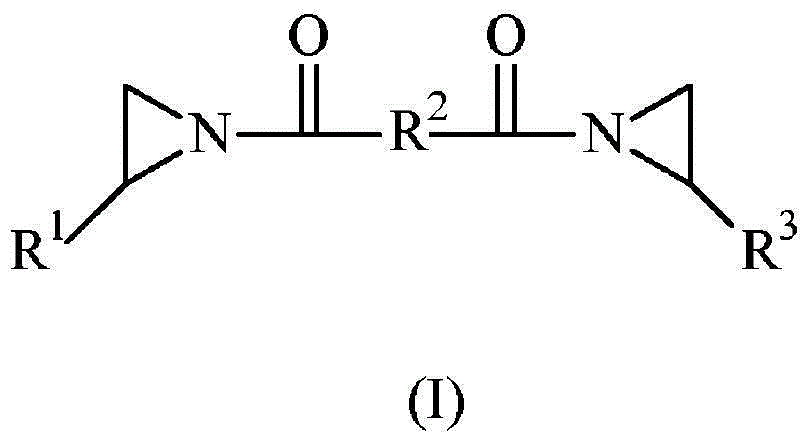
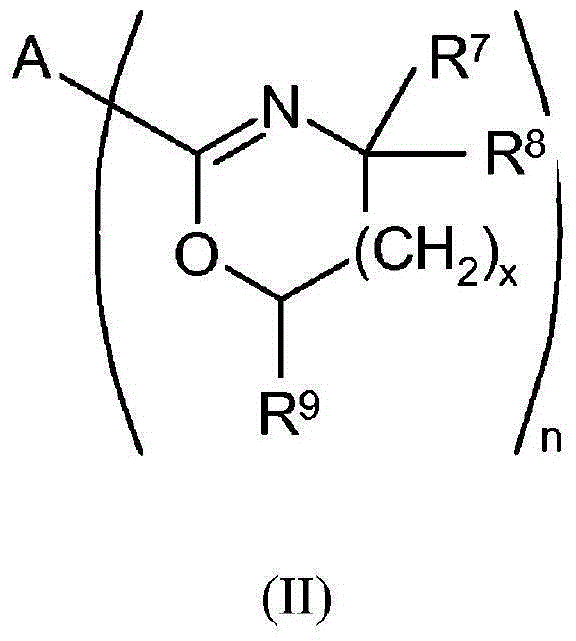
Selective synthesis of 2-octyl acrylate by acid catalyzed esterification of 2-octanol and acrylic acid
A technology of acrylic acid and acid catalyst, which is applied in the preparation of carboxylic acid esters, ester copolymer adhesives, organic chemistry, etc., and can solve problems such as price and supply instability of adhesive products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example
[0117] These examples are for illustrative purposes only, and are not intended to unduly limit the scope of the appended claims. Notwithstanding that the numerical ranges and parameters setting forth the broad scope of the invention are approximations, the numerical values set forth in the specific examples are reported as precisely as possible. Any numerical value, however, inherently contains certain errors necessarily resulting from the standard deviation found in their respective testing measurements. At the very least, and not as an attempt to limit the application of the doctrine of equivalents to the scope of the claims, each numerical parameter should at least be construed in light of the number of reported significant digits and by applying ordinary rounding techniques.
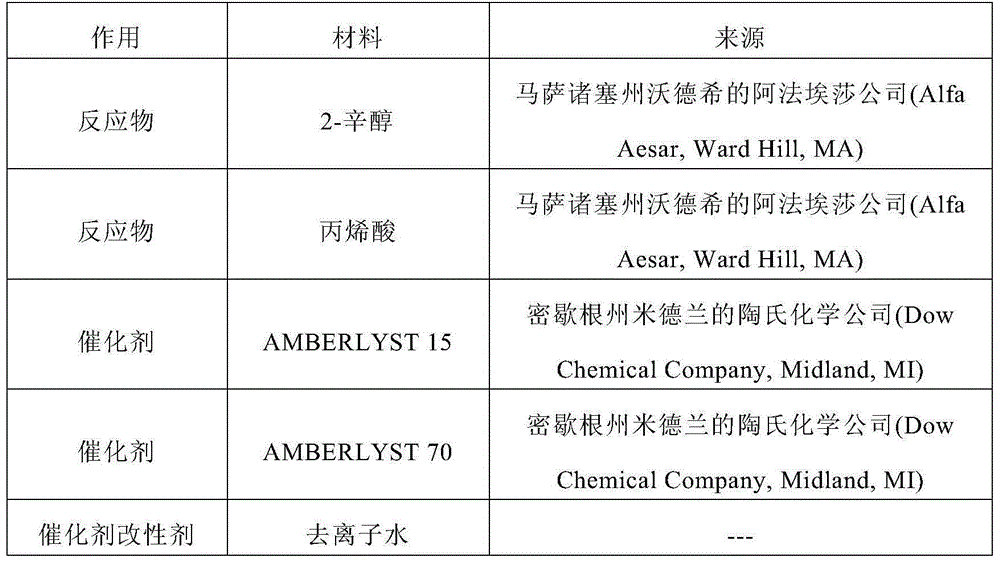
[0118] material overview
[0119] All parts, percentages, ratios, etc. in the examples, as well as in the remainder of this specification, are by weight unless otherwise indicated. Table 1 prov...
example 1
[0124] The 2-octanol conversion, octyl acrylate yield and octene yield of Example 1 were 6.4%, 6.3% and 0.1%, respectively. The 2-octanol conversion, octyl acrylate yield and octene yield of Example 2 were 23.7%, 20.9% and 2.8%, respectively. Octyl acrylate yield is defined as the ratio of the molar flow of octyl acrylate exiting the reactor divided by the molar flow of 2-octanol entering the reactor. Octene yield is defined as the ratio of the molar flow of octene exiting the reactor divided by the molar flow of 2-octanol entering the reactor. The selectivities of the octyl acrylate products of Example 1 and Example 2 were 98.4% and 88.1%, respectively. All results are provided in Table 2 below.
example 3
[0125] Example 3: High yield and selectivity of 2-octyl acrylate
[0126] A 0.5 inch I.D. x 12 inch long stainless steel reactor tube was filled with 20 g of AMBERLY ST 70 catalyst material (sulfonated styrene divinylbenzene copolymer). A 1:1 molar ratio of premixed 2-octanol:acrylic acid (2-octanol derived from castor oil and acrylic acid containing 200 ppm by weight of MEHQ) containing 1% by weight of added water was added at 0.2 mL min -1 Total flow (0.00436molmin -1 or 0.56764gmin -1 2-octanol, 0.00436molmin -1 or 0.31409gmin -1 Acrylic acid, 0.00049molmin -1 or 0.00891gmin -1 water) was continuously fed into the reactor and the reactor pressure was maintained at approximately 50 psig (0.45 MPa). The reactor temperature was maintained at 90°C throughout. After at least three dwells (eg, about 3 minutes total) to reach a steady state, the product was collected for analysis and found to consist primarily of a mixture of octanol, acrylic acid, octene isomers, water, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com