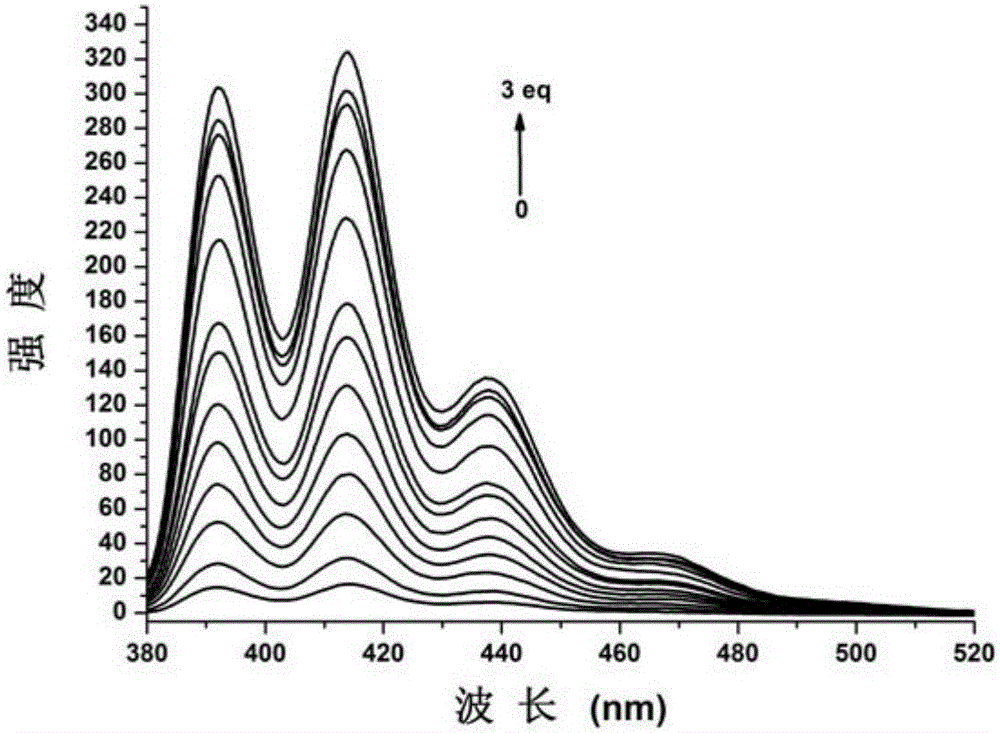
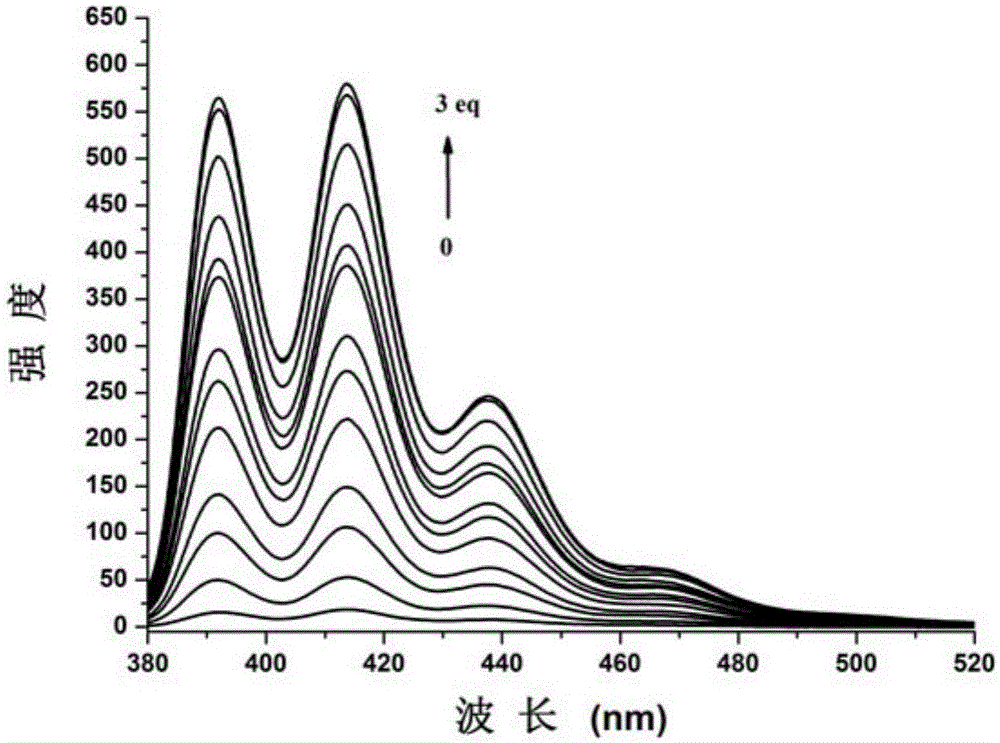
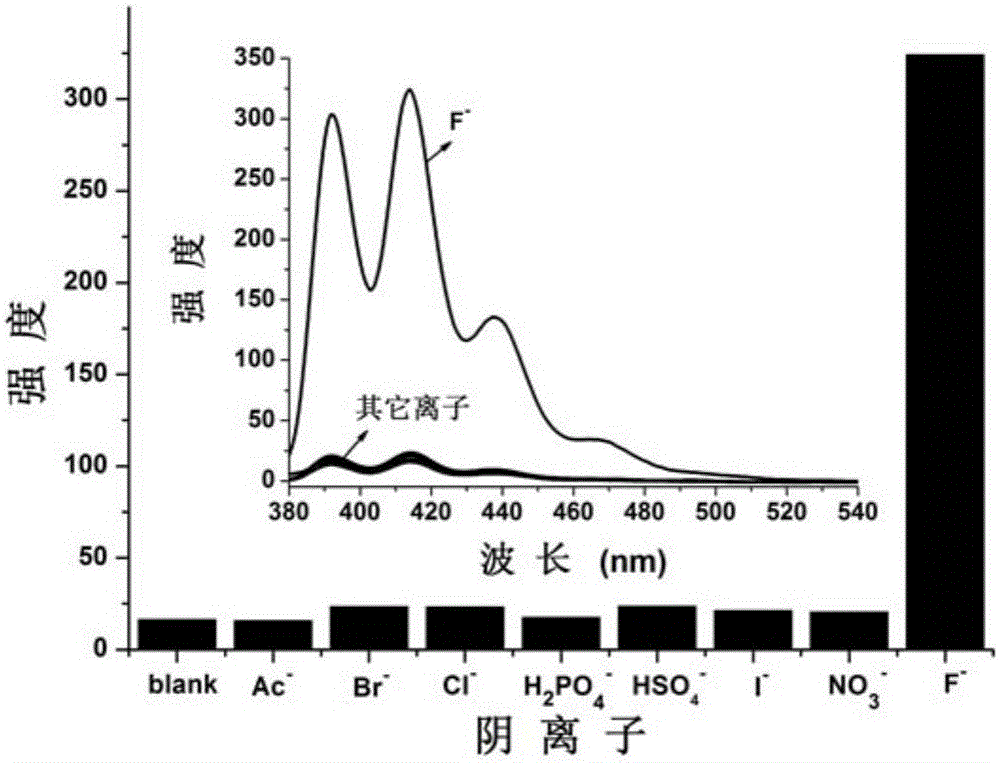
Carboxylate radical bridged binuclear iron-sulfur-cluster fluorescent probe, preparation method and application thereof
一种荧光探针、羧酸根的技术,应用在荧光/磷光、化学仪器和方法、硫化染料等方向,达到操作简单、选择性好、荧光变化敏锐的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] A method for preparing a carboxylate-bridged dinuclear iron-sulfur cluster fluorescent probe, the reaction mechanism is as follows:
[0030]
[0031] (1) Addition reaction
[0032] Addition reaction of 9-anthracenemethylamine and p-methoxycarbonylphenylisocyanate to obtain the corresponding carboxylate;
[0033] (2) Hydrolysis reaction
[0034] Mixing the product solution obtained in step (1) with water and performing a hydrolysis reaction to obtain intermediate 3;
[0035] (3) Neutralization reaction
[0036] The intermediate 3 obtained in step (2) is reacted with a base to obtain a carboxylate;
[0037] (4) Coordination reaction
[0038] The carboxylate salt obtained in step (3) is coordinated with the dinuclear iron precursor 4a or 4b to obtain fluorescent probes 5a and 5b.
Embodiment 1
[0039] Example 1 Synthesis of carboxylate-bridged dinuclear iron-sulfur cluster fluorescent probe 5a
[0040] 0.86g p-methoxycarbonyl phenyl isocyanate was added in 180mL dichloromethane solution containing 9-anthracenemethylamine (1g), stirred at room temperature for 24 hours, filtered, 10mL chloroform washed the filter cake, and then the filter cake was dissolved in ethanol ( 10 mL), 2M sodium hydroxide solution (10 mL) was added thereto, and the temperature was raised to 75° C. to react for 12 hours. Then add 3M hydrochloric acid to the reaction solution to adjust the pH=6, filter with suction, wash the filter cake with 5mL water and ethanol respectively, and finally recrystallize with a mixed solvent of dimethyl sulfoxide-acetone to obtain hydrolyzate 3 (0.92g, 52% ).
[0041] 1 HNMR (400MHz, DMSO-d 6 ):δ11.24(br,1H),8.99(s,1H),8.61(br,1H),8.54(d,J H-H =8Hz,2H),8.11(br,2H),7.72(br,2H),7.54-7.61(m,4H),7.35(br,3H),5.31(br,2H). 13 CNMR (100MHz, DMSO-D 6 ): δ155.08, 141....
Embodiment 2
[0046] Example 2 Synthesis of carboxylate-bridged dinuclear iron-sulfur cluster fluorescent probe 5b
[0047] 4b was used as the dinuclear iron precursor, and the rest of the operation steps were the same as in Example 1 to obtain 5b (105 mg, 58%).
[0048] 1 HNMR (400MHz, CD 2 Cl 2 ):δ8.44(s,1H),8.33(d,J H-H =8Hz,2H),8.01(d,J H-H =8Hz, 2H), 7.46-7.51(m, 4H), 7.14(d, J H-H =8Hz,2H),6.93(d,J H-H =8Hz, 2H), 6.73(s, 1H), 5.33(br, 2H), 5.26(br, 1H), 1.93(t, J H-H =8Hz,6H),1.63(q,J H-H =8Hz,4H),1.46(s,30H).IR(KBr,cm -1 ):3421(m), 3056(w), 2981(m), 2925(s), 1698(s), 1597(s), 1519(s), 1448(w), 1403(s), 1375(m) , 1319(m), 1231(m), 1178(m), 1073(w), 1018(s), 845(s), 778(m), 736(m).
[0049] ESI-HRMS(m / z):[M-PF 6 ] + 873.2506;calcd.valueforC 47 h 57 Fe 2 N 2 o 3 S 2 :873.2509.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com