Methods and compositions for improving cognitive function
A composition and hydrate technology, applied in the direction of drug combination, heterocyclic compound active ingredients, pharmaceutical formulations, etc., can solve the problems of limited usefulness of donepezil
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
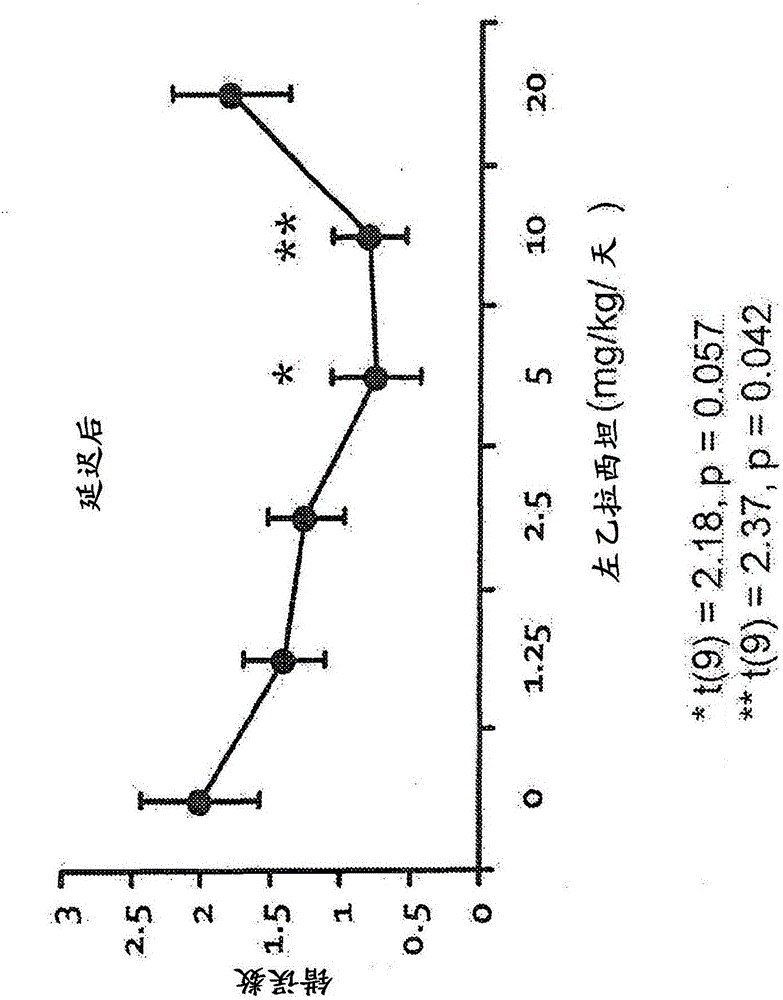
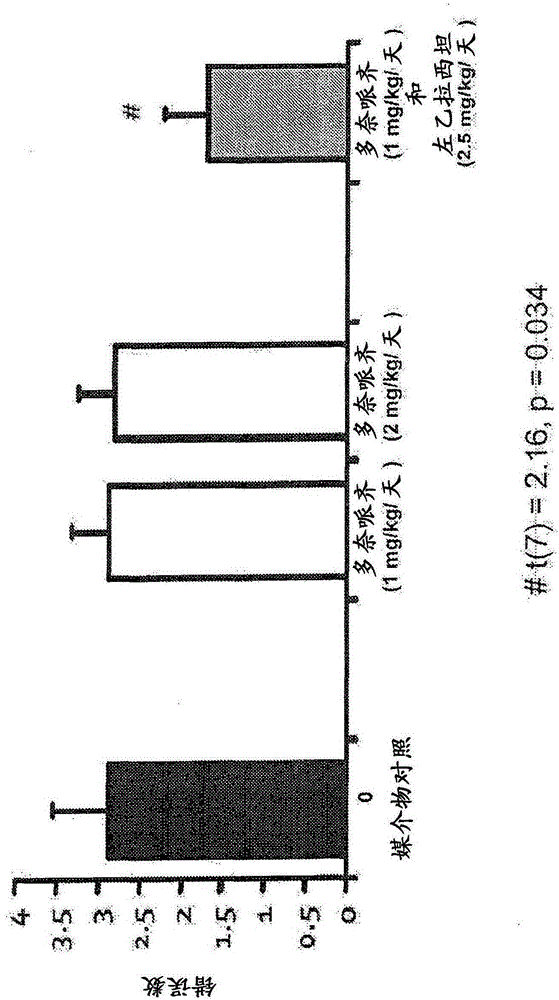
[3846] Example 1: Behavioral evaluation of levetiracetam, donepezil and combination therapy in the radial arm maze task
[3847] Aged, male Long-Evans rats were obtained from Charles River Laboratories (Raleigh, NC) at the age of 8 to 9 months, and were kept in an animal shelter until they were 24 to 26 months old. All rats were individually reared at 25°C and maintained a 12-hour light / dark cycle. Unless otherwise specified, eat and drink at will. The health and pathogen-free status of the rats throughout the experiment were checked, and an autopsy was performed when sacrificed.
[3848] All rats were screened by standardized evaluation of spatial cognition. This background evaluation used the well-established MWM scheme described above. The aged rats that proved to have impaired memory performance in the standardized evaluation of spatial cognition, namely, AI rats, were selected for drug intervention research. Under the following different drug / control treatment conditions, ...
Embodiment 2
[3863] Example 2: Levetiracetam treatment in human subjects with aMCI
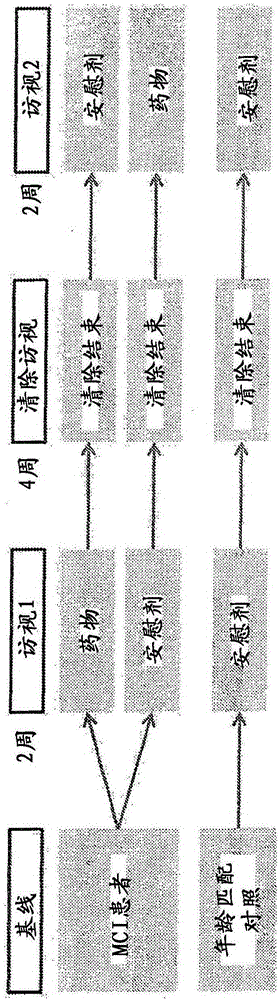
[3864] An 8-week in-subject trial involving 17 low-dose levetiracetam, amnestic MCI (aMCI) subjects and 17 age-matched controls was conducted. During the study period, each aMCI subject received drug and placebo treatment in two cycles of each two weeks, so that the order of treatment was balanced among different aMCI subjects (see image 3 ). An age-matched control subject treated with a placebo was used as another control. Cognitive tests and fMRI imaging data were obtained from the subjects after each two-week cycle of drug / placebo treatment.
[3865] Participants and clinical characterization
[3866] 17 right-handed aMCI patients were recruited from the Alzheimer's Disease Research Center (ADRC) and other referrals at JohnsHopkins Hospital. Another 17 right-handed healthy volunteers were recruited from the control participant pool of ADRC and other referral institutions. Conduct a telephone consultation ...
Embodiment 3
[3894] Example 3: The effect of levetiracetam in subjects with aMCI
[3895] An 8-week in-subject trial involving 38 subjects with amnestic MCI (aMCI) and 17 age-matched controls treated with low-dose levetiracetam. During the study period, each aMCI subject received drug and placebo treatment in two cycles of each two weeks, so that the order of treatment was balanced among different aMCI subjects (see Figure 7 ). An age-matched control subject treated with a placebo was used as another control. Cognitive tests and fMRI imaging data were obtained from subjects after each two-week cycle of drug / placebo treatment.
[3896] Participants and clinical characterization
[3897] 38 right-handed aMCI patients were recruited from the Alzheimer's Disease Research Center (ADRC) and other referrals at Johns Hopkins Hospital. Another 17 right-handed healthy volunteers were recruited from the control participant pool of ADRC and other referral institutions. Conduct a telephone consultation o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com