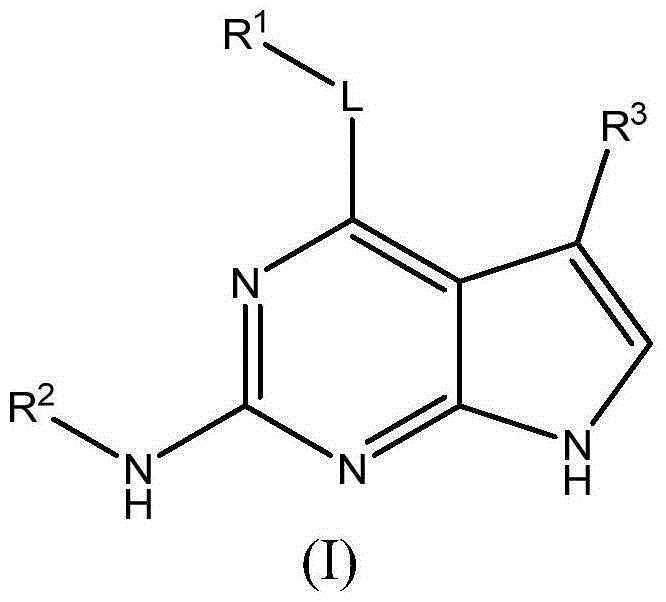
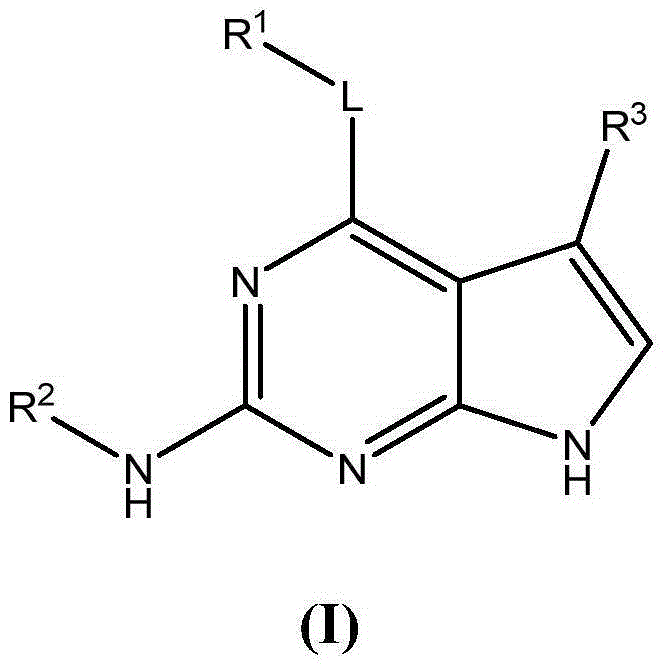
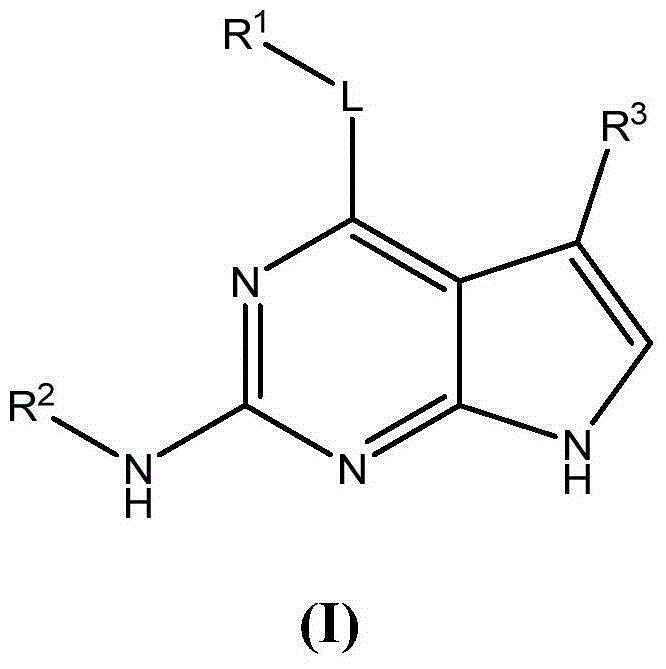
Substituted pyrrolopyrimidine compounds, compositions thereof, and methods of treatment therewith
A technology of compounds and alkyl groups, applied in the direction of drug combination, medical preparations containing active ingredients, organic chemistry, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0266] Example 1: 2,4-dichloro-5-iodo-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine
[0267]
[0268] 2,4-dichloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine. With 2,4-dichloro-7H-pyrrolo[2,3-d]pyrimidine (950g, 5053mmol) and DCM (16 L) to fill a 50-L jacketed reactor. The resulting tan suspension was cooled to 16°C and N-iodosuccinimide (1598 g, 7104 mmol) was added portionwise over 20 minutes. The reaction mixture was stirred at room temperature for 16 hours, after which time TLC analysis (2:1 hexane / ethyl acetate) indicated that the reaction was complete. The resulting precipitate was filtered, washed with DCM (3x1.5L), and dried under reduced pressure at 40°C for 64 hours to afford 1447g (yield: 91%) of the title compound as a beige solid. MS(ESI)m / z314.0[M+1] + .
[0269] 2,4-Dichloro-5-iodo-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine. Flush with nitrogen and wash with 2, 4-Dichloro-5-iodo-7H-pyrrolo[2,3-d]pyrimidine (1437g, 4578mmo...
Embodiment 2
[0270] Example 2: 4-(4-(cyclopentylamino)-5-(4-hydroxyphenyl)-7h-pyrrolo[2,3-d]pyrimidin-2-ylamino)-3-methoxy -n-methylbenzamide
[0271]
[0272] 2-Chloro-N-cyclopentyl-5-iodo-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine-4- Amine. 2,4-dichloro-5-iodo-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine (1 equivalent) , cyclopentylamine (1 equiv), sodium tert-butoxide (7.5 equiv) and 1,4-dioxane (0.28M) were combined in a sealable container with a stir bar. The resulting mixture was placed under a nitrogen atmosphere, sealed, stirred vigorously, and heated at 70°C. After cooling to room temperature, the reaction mixture was directly loaded onto a silica gel column and purified by flash chromatography (Biotage) (0-20% ethyl acetate in hexanes) to give the title compound as a yellow solid (94% yield ). MS(ESI)m / z459.3[M+1] + .
[0273] 4-(2-Chloro-4-(cyclopentylamino)-7-((2-(trimethylsilyl)ethoxy)methyl)-7H-pyrrolo[2,3-d]pyrimidine-5 -yl...
Embodiment 3
[0276]Example 3: 4-((4-(cyclopentyloxy)-5-(2-methylbenzo[d]oxazol-6-yl)-7H-pyrrolo[2,3-d]pyrimidine -2-yl)amino)-3-methoxy-N-methylbenzamide
[0277]
[0278] 2-Methyl-6-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)benzo[d]oxazole. Will be contained in 1,4- A suspension of 6-bromo-2-methylbenzo[d]oxazole (1 eq), bis(pinacolate) diboron (2 eq), potassium acetate (3 eq) in dioxane was treated with argon Degas for 10 minutes. Then, 1,1'-bis(diphenylphosphino)ferrocenepalladium(II) chloride dichloromethane adduct (0.05 equiv) was added and the solution was further degassed with argon for 10 minutes. The reaction mixture was heated at 110°C overnight. The reaction mixture was cooled to room temperature, the solvent was removed under reduced pressure and the crude product was purified by column chromatography (100-200 mesh silica gel; 0-10% ethyl acetate in n-hexane as eluent) to afford the title compound. (Yield: 34%), MS (ESI) m / z 260 [M+1] + .
[0279] 4-Amino-3-methoxy-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com