Site-specific antibody-drug conjugation through glycoengineering
A technology of glycans and linkers, applied in the field of site-specific antibody-drug conjugation through glycoengineering, can solve problems such as coupling difficulties and antibody affinity reduction.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 12C3
[0365] Example 1. Design, preparation and characterization of 2C3 anti-CD-52 highly glycosylated antibody mutants
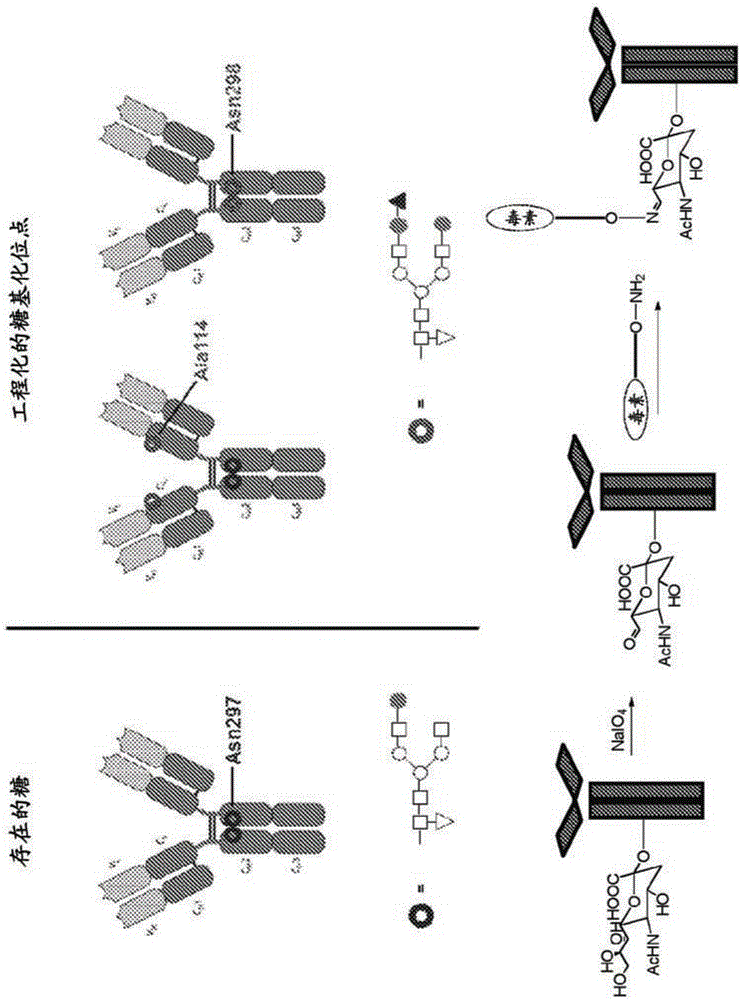
[0366] For the purpose of adding large groups to the interaction interface (e.g. FcRn binding site to modulate antibody pharmacokinetics), for modulating antibody effector functions by altering its interaction with FcγRs, or for introducing novel cross-linking sites Point subsequence chemical modification for conjugation of effector moieties including but not limited to drugs, toxins, cytotoxic agents and radionuclides, multiple hyperglycosylation mutations were designed in the heavy chain of anti-CD-52 antibody 2C3. The highly glycosylated 2C3 mutants are illustrated in Table 3.
[0367] Table 3. Highly glycosylated 2C3 anti-CD-52 mutants
[0368]
[0369] 1A. Generation of hyperglycosylated mutants of 2C3 anti-CD-52 antibody
[0370] The A114N mutation, referred based on the Kabat numbering system, was introduced into the CH1 domain of 2C3 by mutagenesis...
Embodiment 2
[0384] Example 2. Preparation of highly glycosylated mutants in several antibody backbones
[0385] In addition to the 2C3 anti-CD-52 antibody, the A114N mutation was engineered in several other antibody backbones to identify unique hyperglycosylation sites that could be introduced into unrelated heavy chain variable domain sequences. The highly glycosylated anti-TEM1, anti-FAP and anti-Her2 mutants are set forth in Table 5.
[0386] Table 5. A114N and / or S298N mutants designed in several unrelated antibody frameworks
[0387]
[0388] 2A. Generation of anti-TEM1 and anti-FAP antibody hyperglycosylation mutants
[0389] The A114N mutation designed based on the Kabat numbering system was introduced into the anti-TEM1 and anti-FAP CH1 domains by mutagenesis PCR. To generate the full-length antibody, the mutated VH plus residue 114 was inserted by ligation-independent cloning (LIC) into the pENTR-LIC-IgG1 vector encoding antibody CH domains 1-3. The full-length mutants we...
Embodiment 3
[0409] Example 3: Generation of S298N / Y300SFc mutants
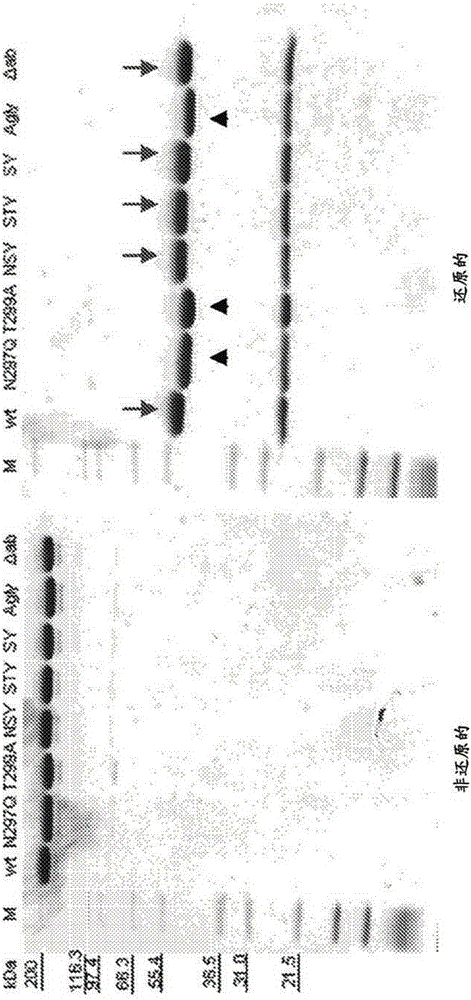
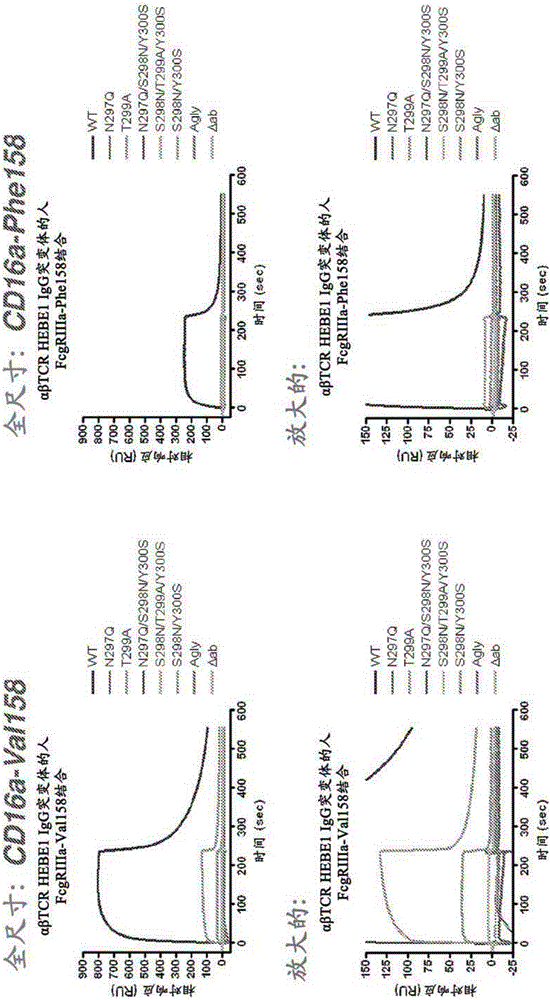
[0410] Engineered Fc variants were designed and generated in which a new glycosylation site was introduced at EU position Ser298, adjacent to the naturally occurring Asn297 site. Glycosylation at Asn297 was maintained or eliminated by mutation. Mutations and expected glycosylation results are set forth in Table 9.
[0411] Table 9: Glycosylation status of various antibody variants
[0412]
[0413] 3A. Generation of altered glycosylation variants of the H66αβ-TCR antibody
[0414]Mutations were performed on the heavy chain of αβ T-cell receptor antibody clone #66 by Quikchange using the pENTR_LIC_IgG1 template. The VH domain of HEBE1ΔabIgG1#66 was amplified with LIC primers, followed by LIC cloning into mutant or wild-type pENTR_LIC_IgG1 to generate full-length mutant or wild-type antibodies. Subcloning was confirmed with DraIII / XhoI double digestion, yielding an approximately 1250 bp-sized insert in successful c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com