Electrochemical synthesis of ammonia in alkaline media
An electrochemical and alkaline technology, applied in the direction of electrodes, electrolytic components, electrolytic process, etc., can solve the problems of high energy consumption and high operating voltage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Example 1: Cell Voltage of Operation
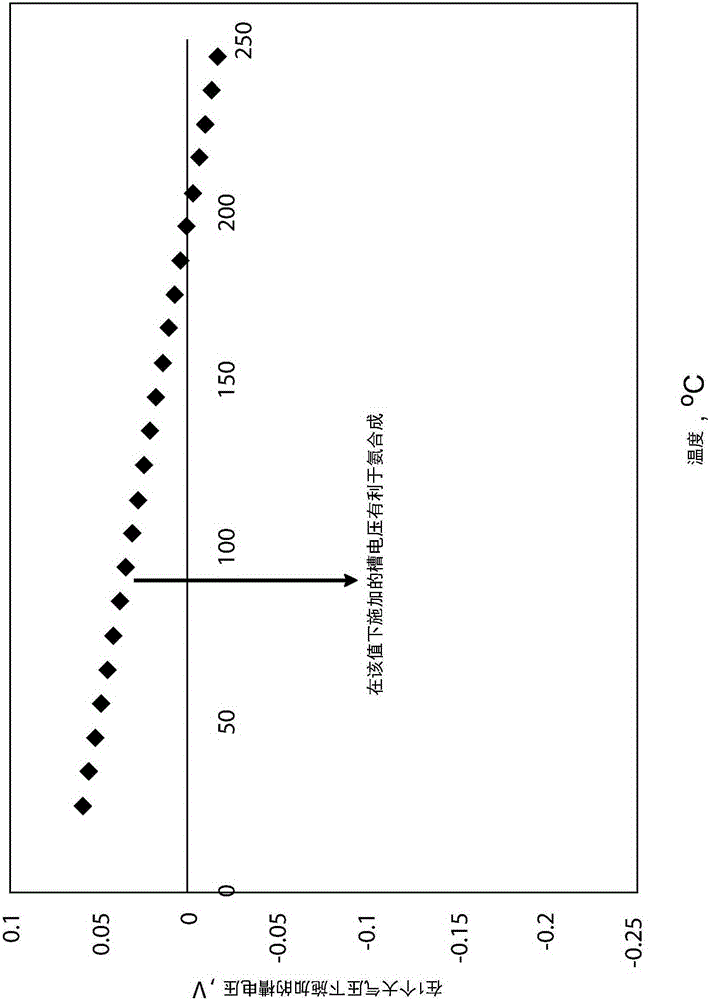
[0052] figure 2 Graph showing theoretically run cell voltage at different temperatures and at 1 atmosphere pressure, which is favorable for ammonia production. like figure 2 As shown in , at temperatures above 195° C., the electrochemical cell 10 transitions from a galvanic cell (positive voltage) to an electrolytic cell (negative voltage). According to an embodiment of the present invention, the potential applied to favor the production of ammonia should be equal to or more negative than the thermodynamic voltage (e.g. figure 2 shown in ). Thus, according to one embodiment, the electrochemical method of forming ammonia includes maintaining a voltage equal to or more negative than a temperature-dependent thermodynamic voltage suitable for producing ammonia. Overpotential (at figure 2 The higher the difference between the thermodynamic potential shown in and the applied cell voltage, the less efficient the induced current...
Embodiment 2
[0053] Example 2: Ammonia Synthesis
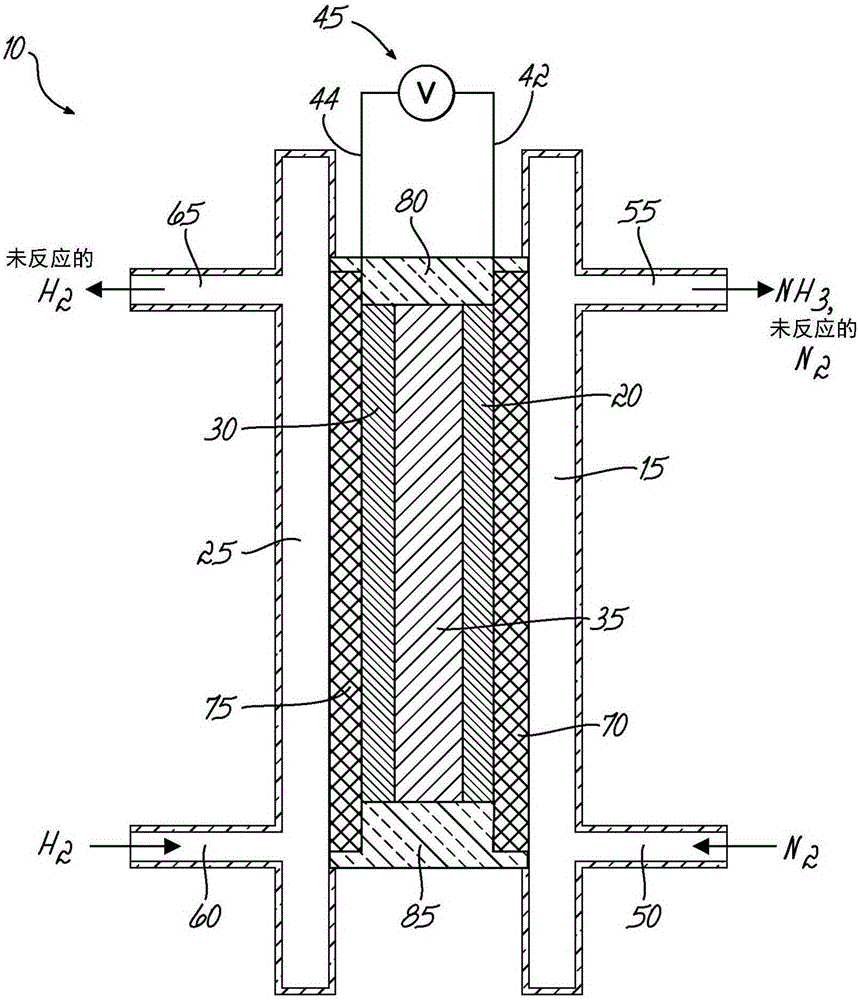
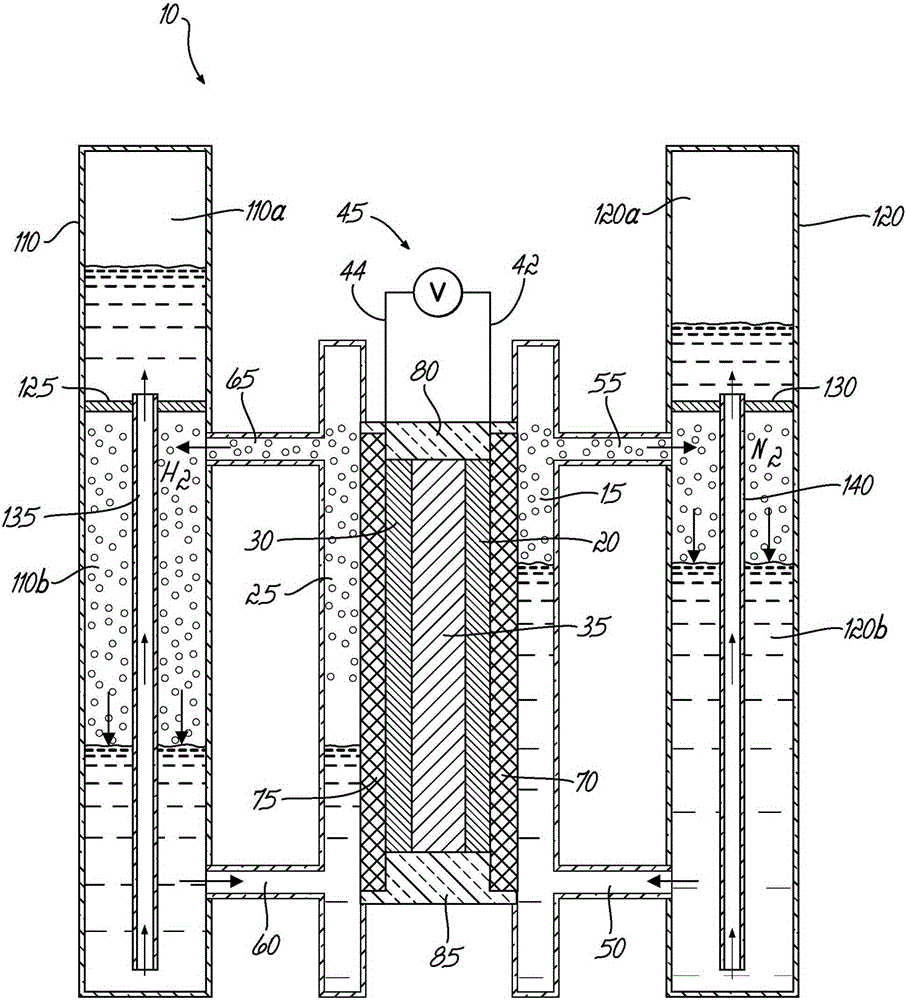
[0054] exist image 3 An electrochemical cell assembly 100 for demonstrating ammonia synthesis according to an embodiment of the invention is shown in . figure 1 The illustrated electrochemical cell 10 may be fluidly coupled to two columns for gas collection by liquid displacement. In this batch configuration, the anode column 110 contains a 5M KOH solution and the cathode column 120 contains 5M KOH / 1MNH 3 The solution. Each column 110 , 120 includes an upper chamber ( 110 a , 120 a ), a lower chamber ( 110 b , 120 b ), and a dividing plate 125 , 130 . The upper chambers (110a, 120a) and lower chambers (110b, 120b) are fluidly coupled to displacement tubes 135, 140, respectively, which allow the transfer of liquid therebetween. The lower chamber 110 b of the anode column 110 is fluidly coupled to the inlet 60 and the outlet 65 . Lower chamber 120b of cathode column 120 is fluidly coupled to inlet 50 and outlet 55 . The cathode elec...
Embodiment 3
[0058] Example 3: Yield and efficiency of induced current
[0059] Based on the current introduced during ammonia synthesis (5mA), the rate of ammonia production was estimated to be 1.06x10 -3 g / h, while the theoretical amount that can be produced based on the consumption of hydrogen in the first 14 minutes of the reaction is 2.98x10 -2 g / h, which represents an ammonia production rate of about 3.5%.
[0060] 1.73x10 -4 mol / sm 2 Ammonia production rate (at Figure 4 at low voltages shown in ) higher than any other value reported in the literature, e.g., 1.13×10 -4 mol / sm 2 , as reported in the following documents: R. Liu, G. Xu, Comparison of Electrochemical Synthesis of Ammonia by Using Sulfonated Polysulfone and Nafion Membrane with Sm 1.5 Sr 0.5 NiO 4 , Chinese Journal of Chemistry 28, 139-142 (2010). The high yields of ammonia observed at the low operating temperatures and pressures of the process of the invention are surprising. The Haber-Bosch process requires 5...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com