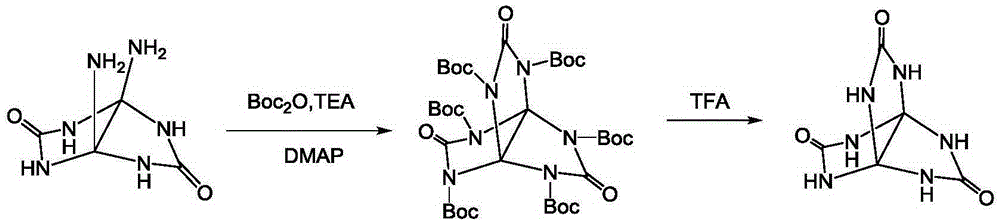
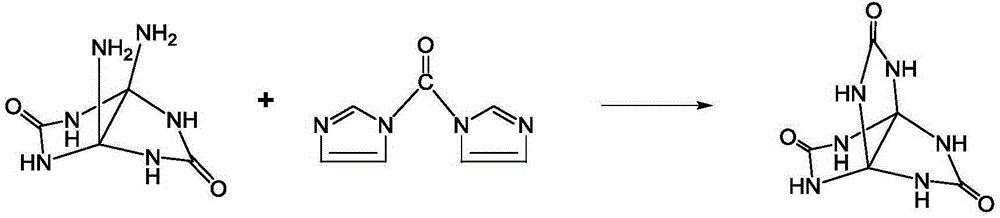
Synthetic method for 3,7,10-trioxo-2,4,6,8,9,11-hexa aza(3,3,3)propellane
A synthetic method, trioxo technology, applied in the direction of organic chemistry, can solve the problems of complex operation process, many reaction steps, low total yield, etc., and achieve the effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0017] Add 35.1mmol diaminoglycoluril and 40.36mmol N,N'-carbonyldiimidazole to 120ml dimethyl sulfoxide under stirring at a temperature of 20°C. After the addition, react for 60 hours, pour the reaction solution into acetone, and filter , the filter cake was washed with methanol, and dried to obtain 5.95 g of 3,7,10-trioxo-2,4,6,8,9,11-hexaaza[3,3,3]propeller, yield 85.6 %.
[0018] Structure Identification:
[0019] Infrared spectrum: IR (KBr) ν: 3218, 2830, 1747, 1688, 1524, 1471cm -1 ;
[0020] NMR spectrum: 1 HNMR (DMSO-d 6 ,500MHz), δ: 8.06(s,6H);
[0021] 13 CNMR (DMSO-d 6 ,125MHz), δ: 159.4,85.2;
[0022] MS:199(M+)
[0023] Elemental analysis: Molecular formula: C 5 h 6 N 6 o 3
[0024] Theoretical value: C30.31, H3.05, N42.41
[0025] Measured values: C29.94, H3.42, N41.98.
[0026] The above structural identification data confirm that the substance obtained in this step is indeed 3,7,10-trioxo-2,4,6,8,9,11-hexaaza[3,3,3]propellerane.
Embodiment 2
[0028] Add 35.1mmol diaminoglycoluril and 36.86mmol N,N'-carbonyldiimidazole to 108ml dimethyl sulfoxide under stirring at a temperature of 20°C. After the addition, react for 70 hours, pour the reaction solution into acetone, and filter , the filter cake was washed with methanol and dried to obtain 5.93 g of 3,7,10-trioxo-2,4,6,8,9,11-hexaaza[3,3,3]propeller, yield 85.3 %.
Embodiment 3
[0030] Under stirring, at a temperature of 15°C, add 35.1mmol diaminoglycoluril and 36.86mmol N,N'-carbonyldiimidazole to 150ml dimethyl sulfoxide respectively. After the addition, react for 50 hours, pour the reaction solution into acetone, and filter , the filter cake was washed with methanol, and dried to obtain 5.91 g of 3,7,10-trioxo-2,4,6,8,9,11-hexaaza[3,3,3]propeller, yield 85.1 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com