Benzimidazole derivatives and uses thereof
一种药物、组合物的技术,应用在苯并咪唑衍生物及其用途领域,能够解决限制先导化合物进展、阻碍早期临床评价等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0287] Embodiment 1. The preparation of compound
[0288] Compounds of formula (I) can be prepared by the synthetic sequence shown in Scheme 1 below. Alternatively, compounds of formula (I) may be synthesized by other methods described herein.
[0289]
[0290] Scheme 1. Exemplary Synthesis of Compounds of Formula (I)
[0291] Compound ABI in the initial screen was purchased from ChemDiv (K783-0286). The following syntheses were carried out according to the scheme below.
[0292] O-(benzotriazol-1-yl)-N,N,N',N'-tetramethyluronium hexafluorophosphate (HBTU, 99%) was purchased from Oakwood Products, Inc., and iron powder (99 %, 325 mesh) was purchased from Acros Organics. All other starting materials and solvents were purchased from Aldrich Chemical Co. or Alfa Aesar, and all reagents were used as received. The mixture was purified by flash chromatography using Silicycle SiliaFlashP60 (230-400 mesh) silica gel. All new compounds start with 1 HNMR, 13 Characterized by ...
Embodiment 2
[0303] Example 2. High Throughput Small Molecule Screening
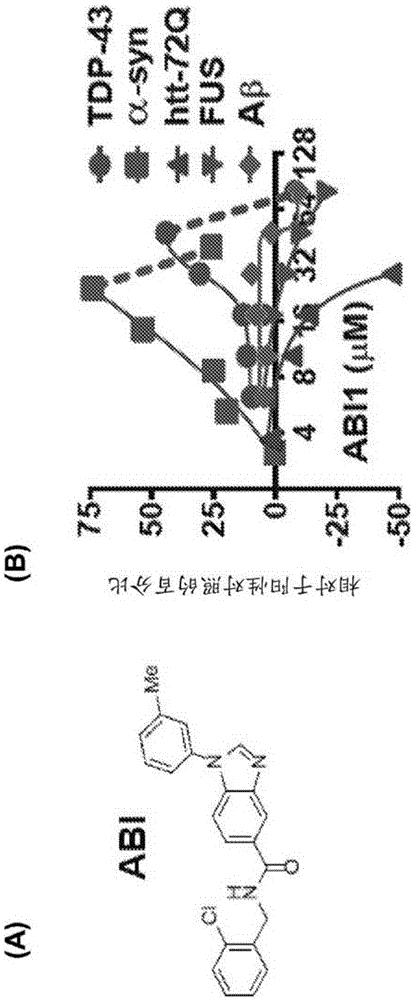
[0304] Taking advantage of the ease of yeast culture and the ability to simultaneously induce the expression of toxic proteins that strongly inhibit growth, 190,000 different compounds were screened for restoring the growth of cells expressing the ND-associated protein TDP-43 (6). Compound ABI (FIG. 1A), identified as having modest potency against TDP-43, was demonstrated to be more potent and efficacious against α-syn (FIG. 1B). However, ABI was not effective against other ND-associated proteins, including htt72Q, FUS / TLS and Aβ peptide (Fig. IB). Thus, rather than targeting a common feature of misfolded protein toxicity, rescue reflects the targeting of ABI (and the ability to reduce TDP-43 levels) to specific α-syn-associated pathologies.
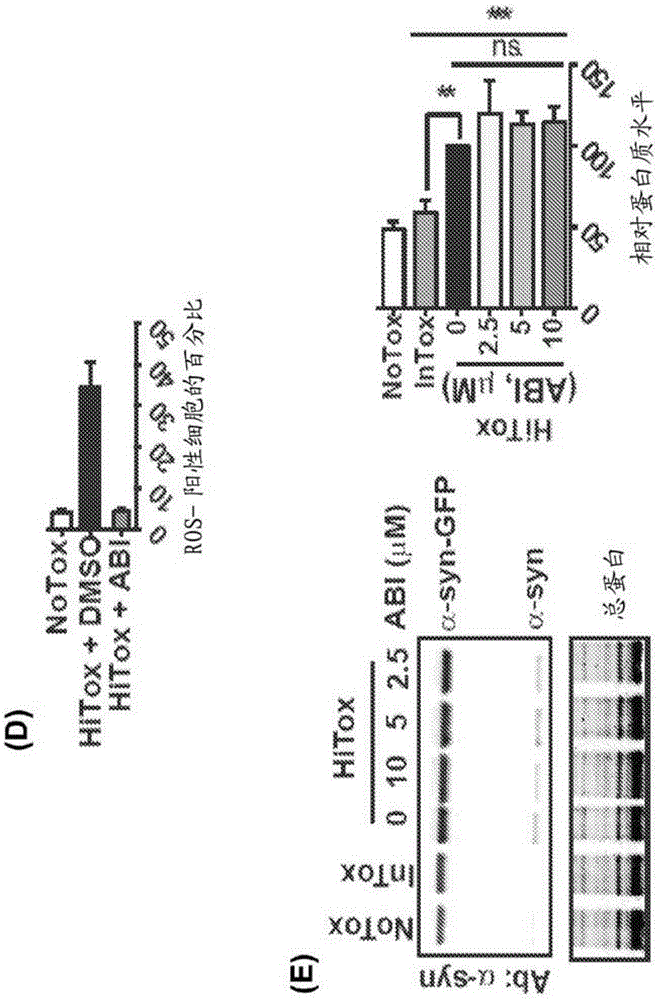
[0305] ABI was found to be able to reverse several key pathogenic features of α-syn toxicity. First, ABI prevents the accumulation of cytoplasmic α-syn foci, which are the ac...
Embodiment 3
[0306] Example 3. Screening of chemical genes
[0307] overexpression screening
[0308] Overexpression screening was performed by using pooled FlexGene libraries (35) transformed into WT yeast impairing drug efflux. Frozen aliquots of pooled yeast stocks were thawed in SGalUra supplemented with 30 mL of 0.05% glucose and grown at 30°C for approximately 8 hours. During this period, the medium was increased approximately 0.5-1 fold. Then, dilute the yeast to OD in SGalUra 600 is 0.0005, which corresponds to approximately 20-fold coverage of diversity in the library and 40 μM ABI2. After approximately 3 days of growth, apparent colony growth in 384-well plates was restored and verified individually. Plasmids were recovered and amplified in E. coli and sequenced by using the Yeast Plasmid Isolation Kit (ZymoResearch). The resulting sequence was then queried with BlastN (NCBI) and a reliable ORF was confirmed.
[0309] Transposon (Tn7) Screening
[0310] Screening based on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com