Detection technology based on pyrosequencing technique for TB (mycobacterium tuberculosis) drug resistant genes
A technology of mycobacterium tuberculosis and pyrosequencing, applied in the direction of recombinant DNA technology, microbial determination/testing, DNA/RNA fragments, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0106] Example 1: Pyrosequencing using the sequencing primers of the present invention
[0107] Materials and methods
[0108] 1.1 Materials
[0109] Plasmids TBIKR (inhA-katG-rpoB gene wild-type plasmid, SEQ ID NO: 27) and TBIKR-m (inhA-katG-rpoB gene mutant plasmid, SEQ ID NO: 28) were synthesized by Invitrogen.
[0110] Table 1: Materials and sources used in the present invention
[0111] material name source Dosage unit PCR reaction solution 1-3 (containing primers, dNTP, MgCl 2 , buffer, etc.) self-provided 27.6 µl dNTP Roche 0.24 µl MgCl 2 PG 2.4 µl 20X buffer QIAGEN 1.5 µl PCR Primer Sets 1-3 Invitrogen 0.09 µl EvaGreen Biotium 0.75 µl HS Taq QIAGEN 0.4 µl Sequencing primers 1-5 Invitrogen 0.8 µl PyroMark Gold Q24 Reagents QIAGEN 1 serving
[0112] 1.2 Equipment
[0113] QIAGEN Rotor-geneQPCR instrument and PyroMark? Q24MDx sequencer.
[0114] ...
Embodiment 2
[0131] Example 2: Comparison of Different Sequencing Primers
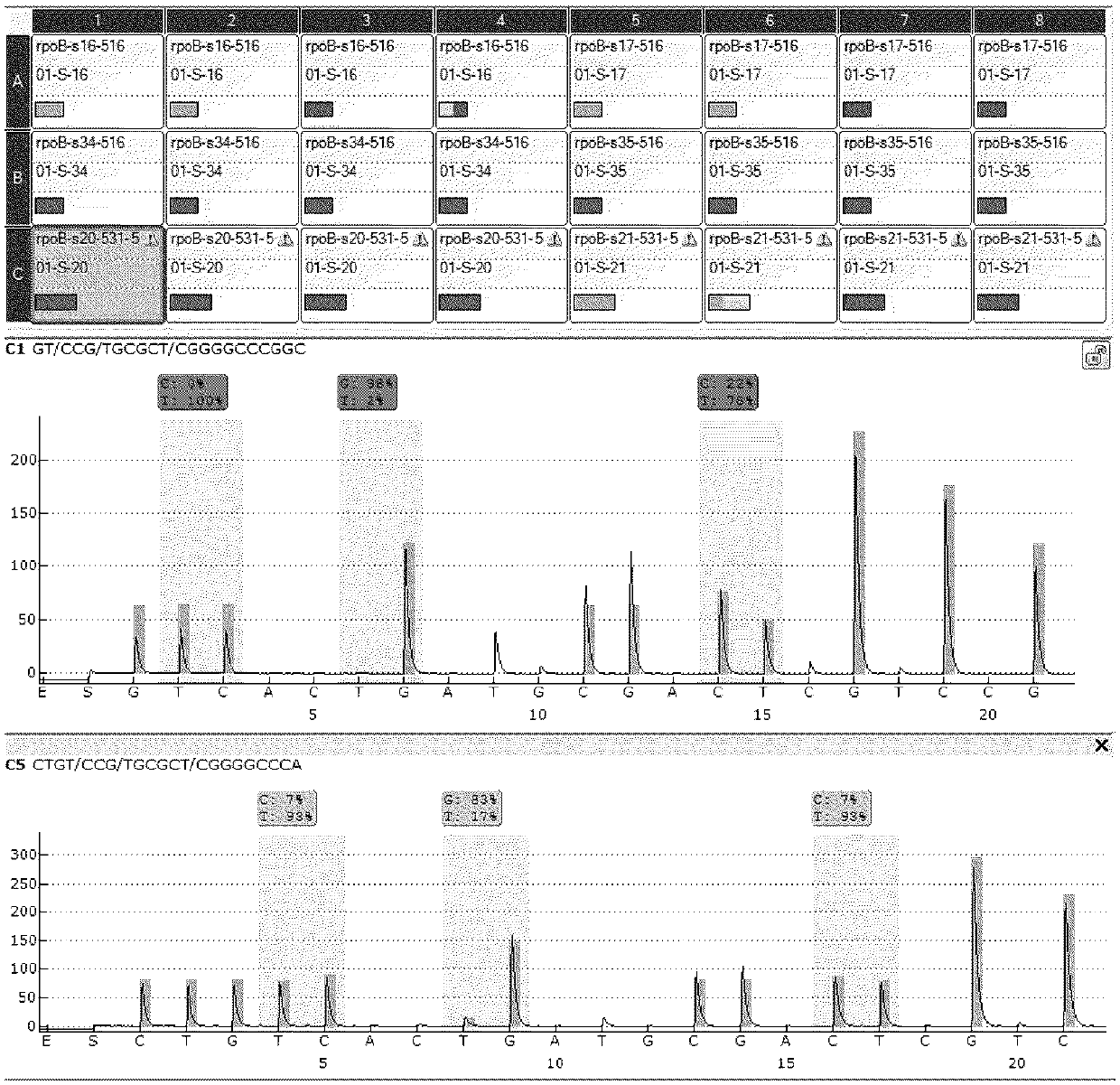
[0132] The materials, instruments, methods and conditions used in this example are the same as in Example 1, but during the pyrosequencing process, the two sequencing primers shown in Table 4 below were used to sequence the mutation in rpoB531533
[0133] Sequencing primers Sequence (5'—3') 01-S-20 CCACAAGCGCCGA (SEQ ID NO: 29) 01-S-21 CAAGCGCCGA (SEQ ID NO: 23)
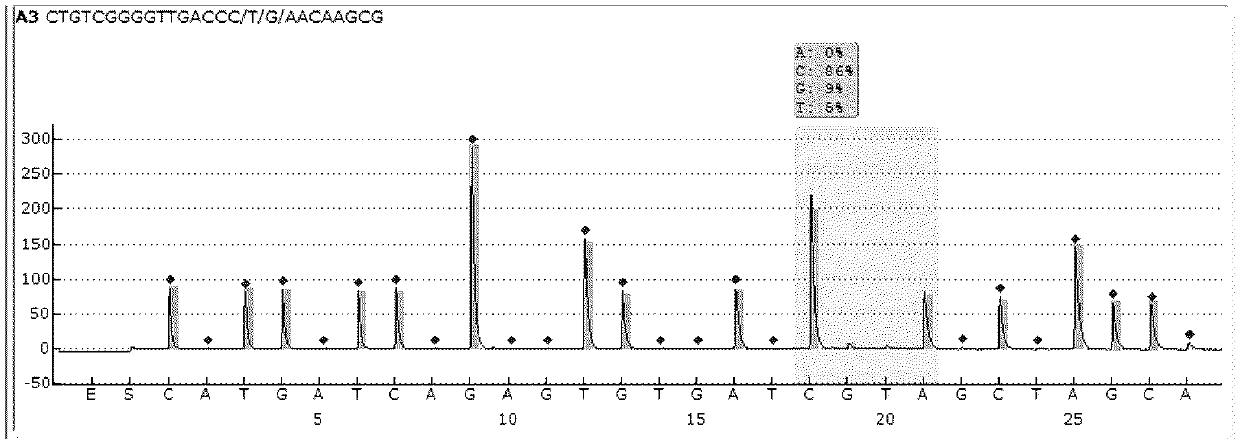
[0134] For sequencing results, see Figure 8 . From Figure 8 It can be seen that although the sequencing primer 01-S-20 is very similar in sequence to the sequencing primer 01-S-21 of the present invention (ie, sequencing primer 5), the sequencing specificity for the same sample (rpoB gene wild-type plasmid sample) is different , the specificity of the sequencing primer 01-S-20 is obviously inferior to that of the sequencing primer of the present invention, and the sequencing background is relatively high.
Embodiment 3
[0135] Example 3: Sequencing of samples from clinical sources with sequencing primers of the present invention
[0136] The materials, instruments, methods and conditions used in this example are the same as those in Example 1, but the sequenced samples are hospital clinical sputum samples containing Mycobacterium tuberculosis rather than wild-type and mutant plasmids. The clinical sputum Samples included resistant and non-resistant sputum samples. The clinical sputum sample was fully liquefied with NaOH, and then the nucleic acid was extracted with a conventional DNA extraction solution (containing tris, NP40 and proteinase K), and then PCR amplification and pyrosequencing of the sample were performed according to Example 1.
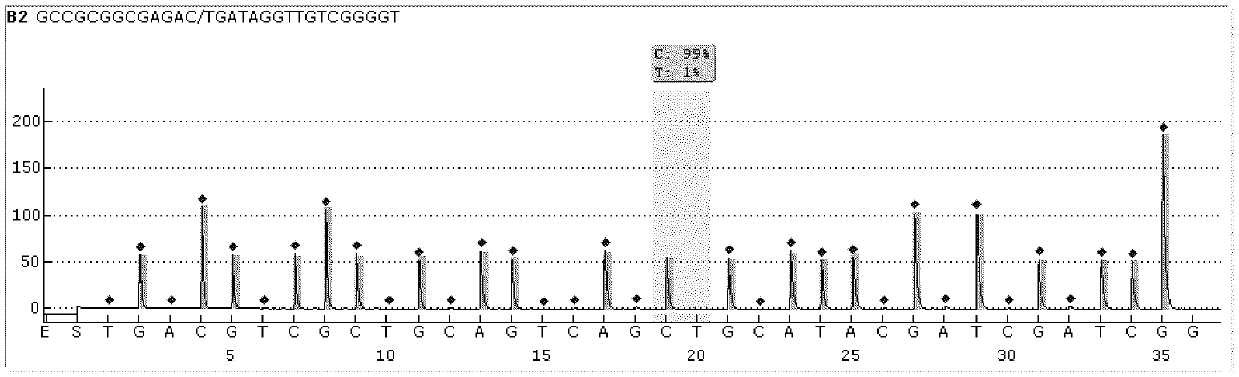
[0137] The sequencing results of each sequencing primer shown in Table 2 of the present invention to the katG315 gene, rpoB526 gene and rpoB531-533 gene of drug-resistant and non-drug-resistant sputum samples are shown in Figure 9 .
[0138] From ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com