Impurity detection method of clarithromycin
A clarithromycin and detection method technology, which is applied in the detection field of impurities in clarithromycin raw materials, can solve the problems of inability to distinguish the positioning requirements of impurities, impurities cannot be attributed, and impurities cannot be separated, so as to achieve drug safety and good economic benefits , the effect of reducing the load
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] The newly developed assay method for clarithromycin-related substances complies with the standards of the Institute for Drug Control, and the assay results are accurate, reliable, and reproducible. See Listing 5 for detection.
[0062] Table 5 Summary of verification results of clarithromycin related substances determination method
[0063]
Embodiment 2
[0065] Determination of clarithromycin API produced by our company
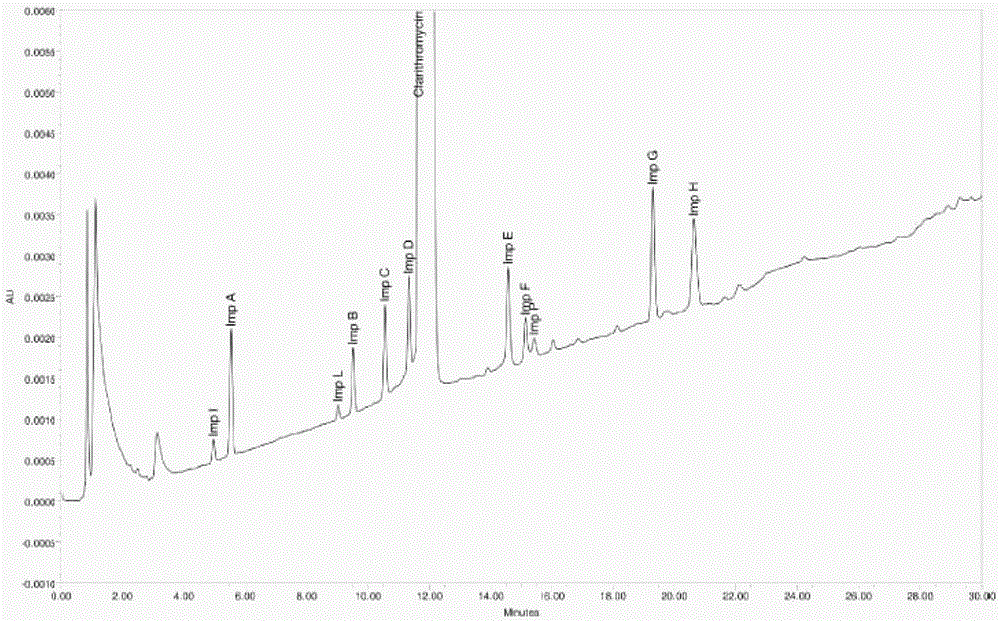
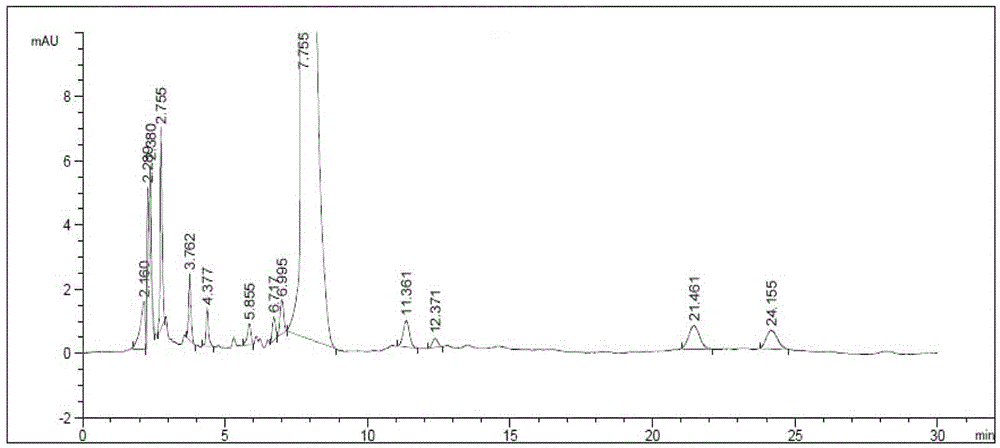
[0066] Get 3 batches of clarithromycin raw materials produced continuously according to the operation steps under the above-mentioned content of the invention to prepare a measurement solution according to law, prepare the requirements under the instrument and chromatographic conditions, measure according to law, and record the chromatogram (see the production of clarithromycin by our company. Vegetarian Raw Drug Representative Figure 4 ), and calculate the result list 6
[0067] List 6
[0068]
[0069]
Embodiment 3
[0071] Determination of clarithromycin tablets produced by our company
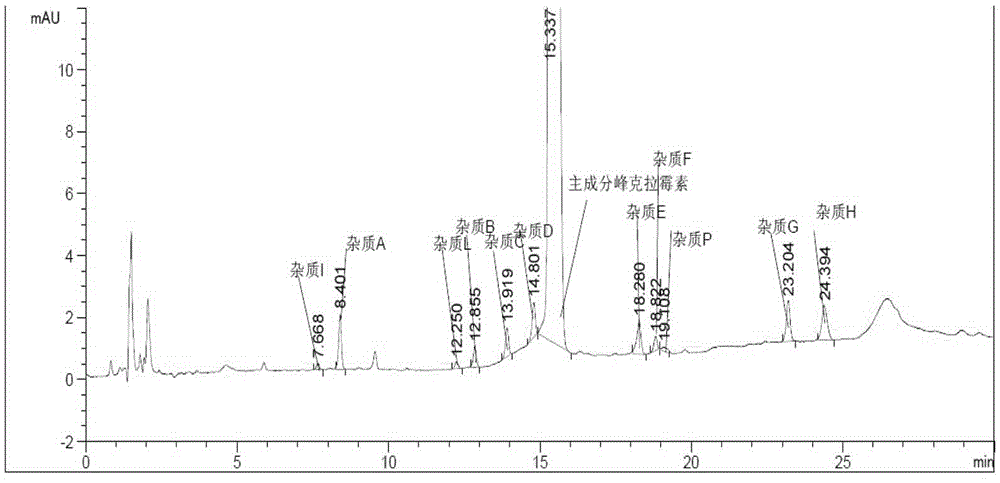
[0072] Get 3 batches of clarithromycin tablets produced continuously according to the operation steps under the above-mentioned content of the invention to prepare the measurement solution according to law, prepare the requirements under the instrument and chromatographic conditions, measure according to law, and record the chromatogram (see the clarithromycin produced by our company. Plain representative Figure 5 ), and the calculation results are shown in List 7
[0073] List 7
[0074]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Elution gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com