IgE antibodies for the inhibition of tumor metastasis
A tumor-associated antigen and antibody technology, applied in the direction of antibodies, anti-tumor drugs, drug combinations, etc., can solve short-term problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0053] For the production of monoclonal antibodies, any technique for producing antibody molecules by passaged cell lines in culture can be used (see, e.g., Antibodies--A Laboratory Manual, Harlow and Lane, eds., Cold Spring Harbor Laboratory Press: Cold Spring Harbor, New York, 1988). These include, but are not limited to, hybridoma technology originally developed by Kohler and Milstein (1975, Nature 256:495-497), and trioma (trioma) technology, human B cell hybridoma technology (Kozbor et al., 1983, Immunology Today, 4:72 ) and EBV-hybridoma technology to produce human monoclonal antibodies (Cole et al., 1985, in Monoclonal Antibodies and Cancer Therapy, Alan R. Liss, Inc., pp. 77-96). In an additional embodiment of the invention, monoclonal antibodies can be produced in germ-free animals using state-of-the-art technology (PCT / US90 / 02545). According to the present invention, a human antibody can be used, and the human antibody can be obtained by transforming human B Cells (...
Embodiment 1
[0100] Example 1: Formation of chimeric IgE gene carrier
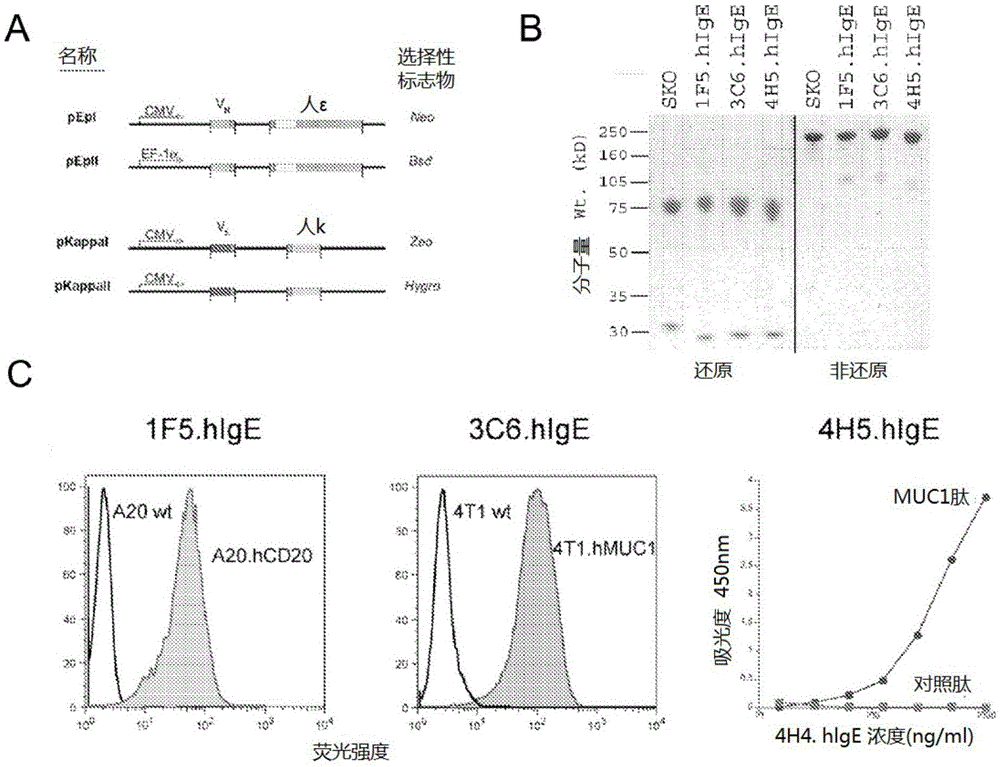
[0101] Hybridomas VU-3C6 and VU-4H5 were generated against two different isoforms of human mucin 1 (hMUC-1 ), a mucin overexpressed on tumors derived from glandular epithelium. Antibody variable gene segments were cloned from each hybridoma and sequenced, then grafted onto human kappa light chain and epsilon heavy chain gene segments using standard procedures. For kcDNA clones obtained from human peripheral lymphocytes the sequence was then compared with database sequences (eg GenBank: J00241.1). The ε constant region cDNA was cloned from an IgE-expressing hybridoma (SKO-007, ATCCCRL8033-1), and then compared with the genome sequence in a database (eg GenBank: J00222.1). The final IgE mouse-human chimeric antibodies were named 3C6.hlgE and 4H5.hlgE. The final plasmid is in figure 1 Shown in A.
[0102] The 1F5 hybridoma targets human CD20 (hCD20), a pan-B cell marker and an important therapeutic target for the trea...
Embodiment 2
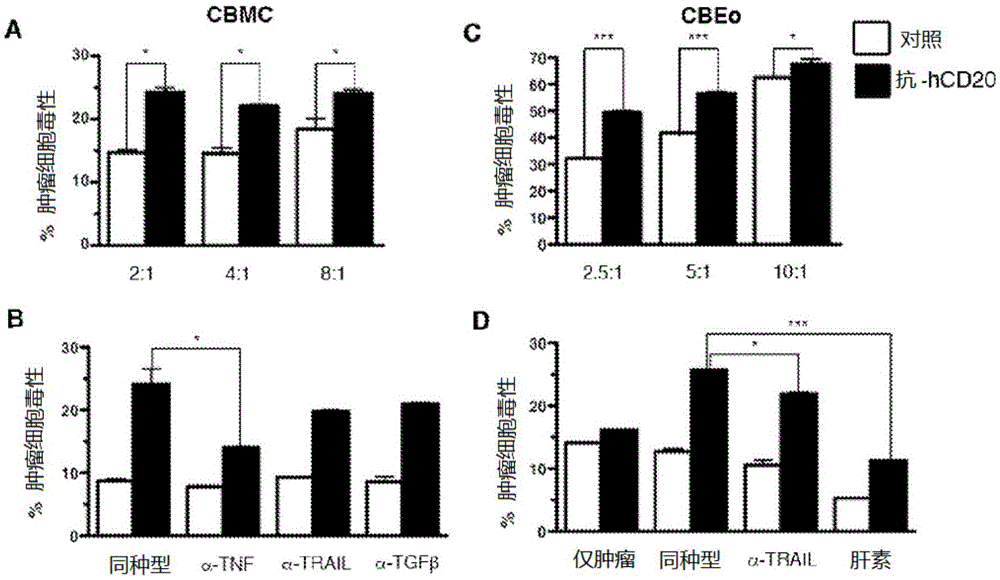
[0105] Example 2: IgE produced by 1F5.hIgE (anti-hCD20) mediates tumor cytotoxicity
[0106] To determine whether the anti-hCD20 chimeric antibody 1F5.hIgE possesses a functional IgEFc region, we investigated its ability to activate cord blood-derived mast cells (CBMCs). Using interleukin-8 (IL-8) production as a measure of CBMC activation, we observed that: CBMCs precoated with 1F5.hIgE were in the presence of hCD20-transfected mouse B cells (A20.hCD20) but not untransfected cells produced IL-8. Similarly, only CBMCs coated with anti-hCD20 IgE (1F5.hIgE) were sensitive to hCD20 + Human B cells OCI-Ly8 responded, whereas CBMCs coated with control IgE (SKO) did not produce IL-8 under these conditions. These data suggest that 1F5.hIgE activates mast cells in an antigen-dependent and antigen-specific manner.
[0107] Mast cell and IgE-mediated tumor cytotoxicity
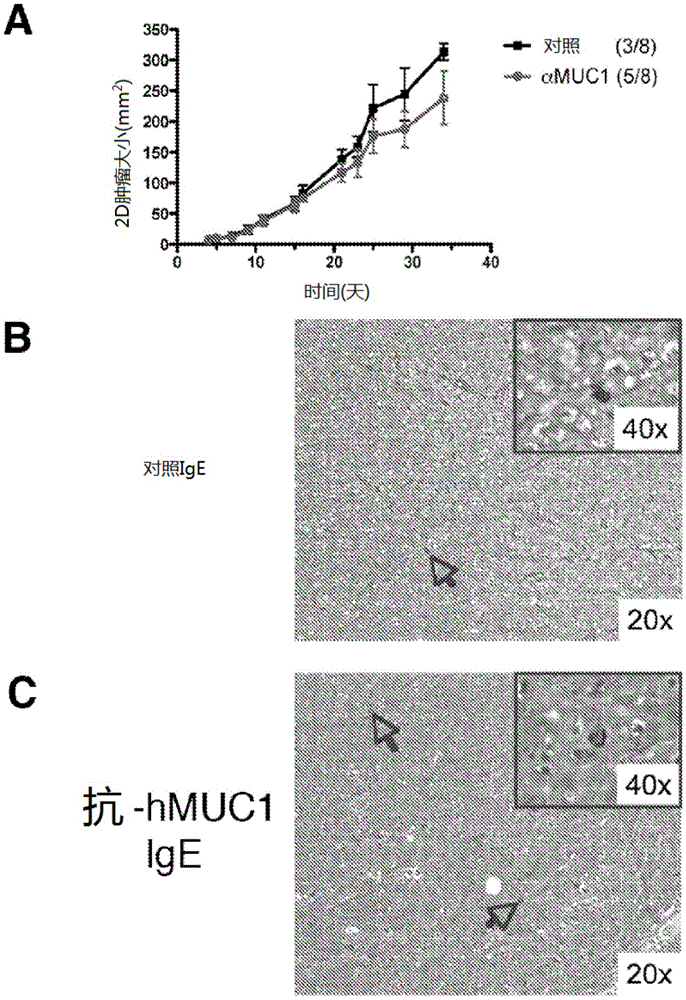
[0108] To test potential tumor-specific IgE-mediated cytotoxic effects, we focused on effector cells known to exp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com