Patents
Literature
134 results about "Metastatic carcinoma" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Metastatic carcinoma is able to grow at sites distant from the primary site of origin; thus, dissemination to the skin may occur with any malignant neoplasm, and these infiltrates may result from direct invasion of the skin from underlying tumors, may extend by lymphatic or hematogenous spread, or may be introduced by therapeutic procedures. The most common malignancy found in bone is metastatic carcinoma.
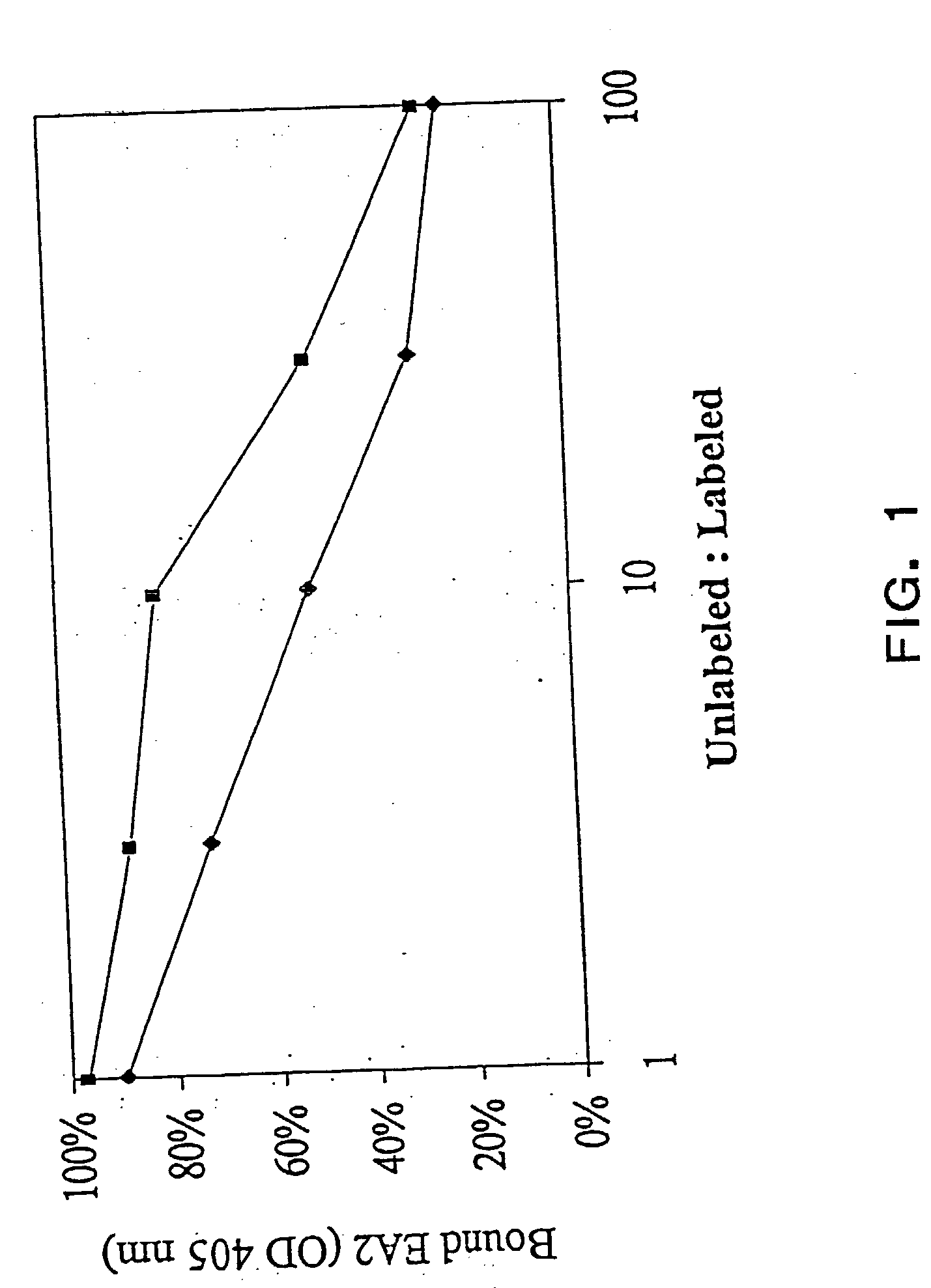
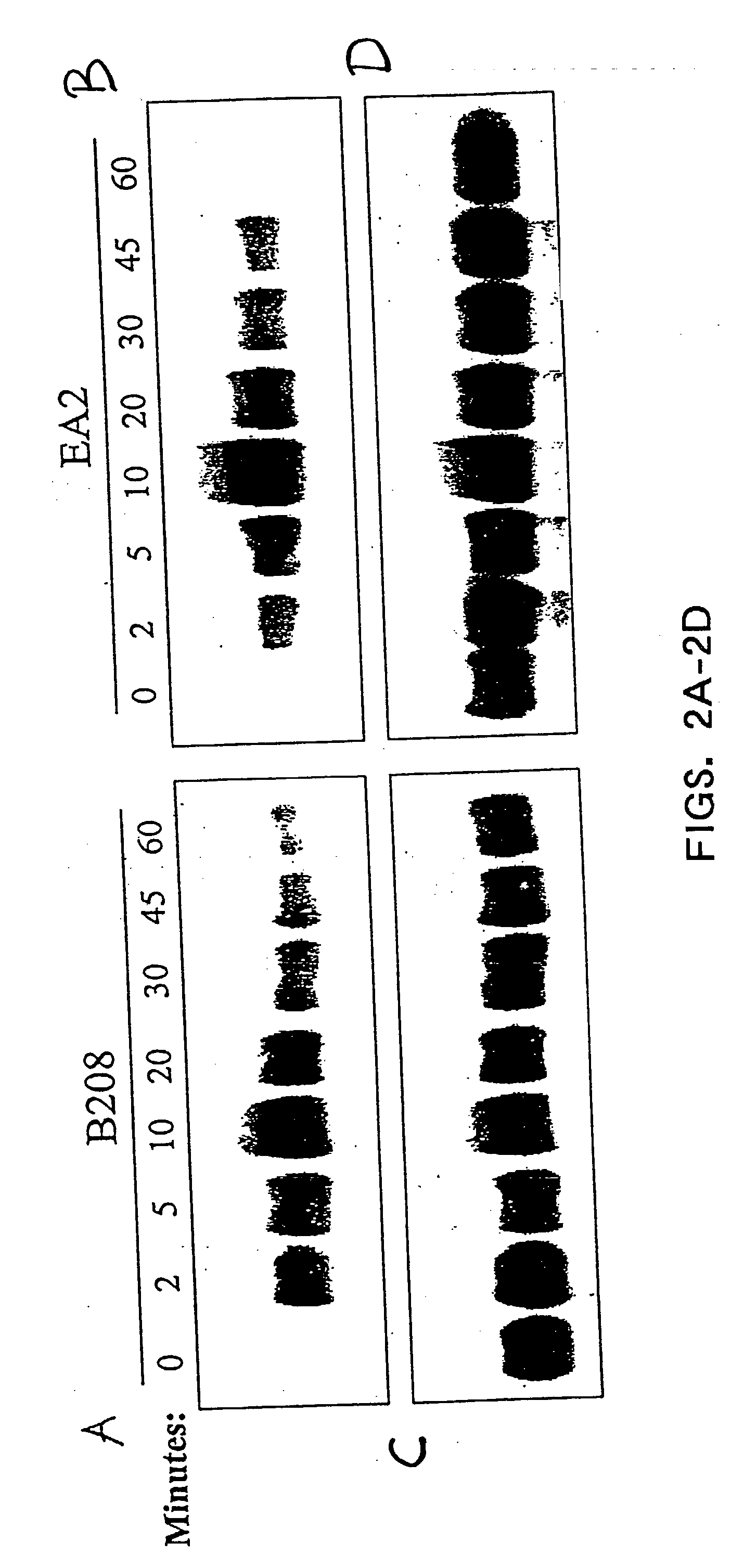
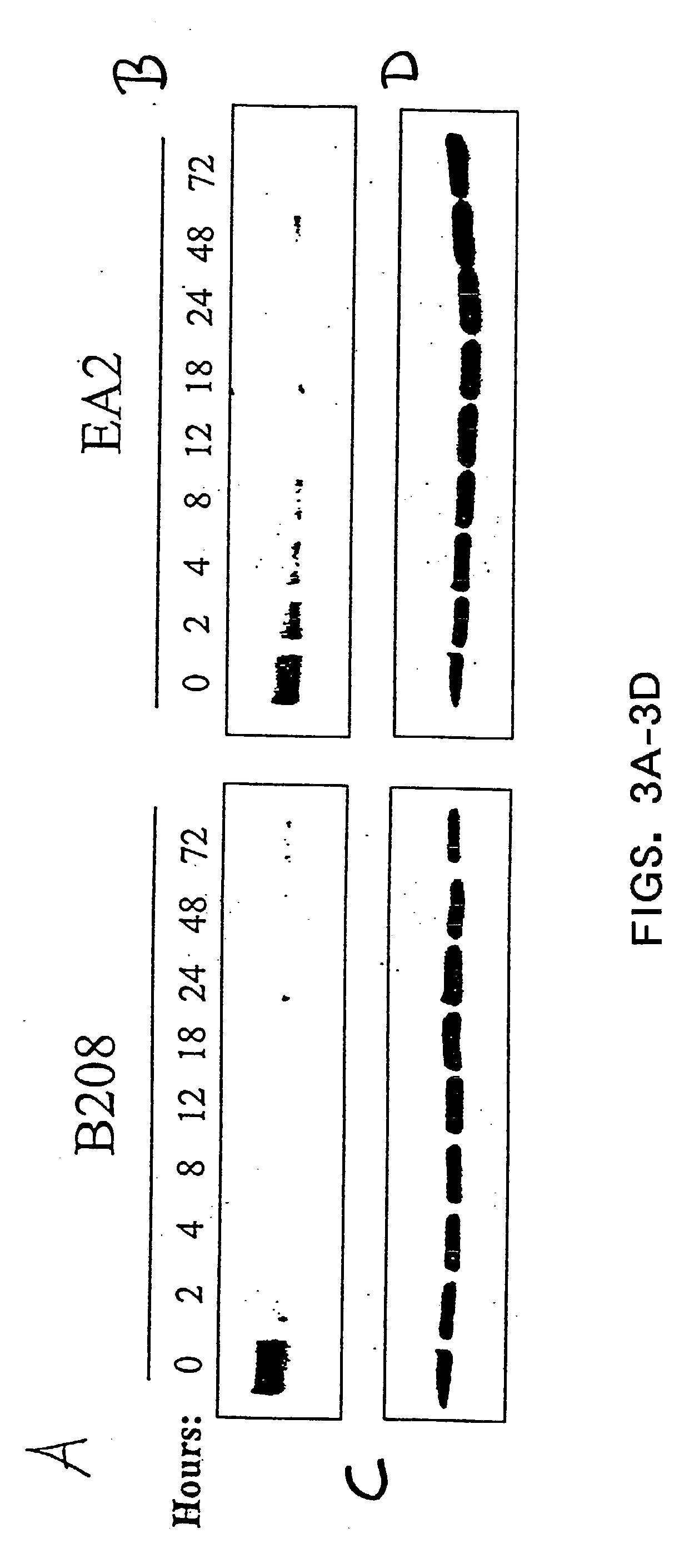
EphA2 monoclonal antibodies and methods of use thereof
InactiveUS20040028685A1Decrease contactStable interactionOrganic active ingredientsFungiTubular networkLymphatic Spread
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and agonizes EphA2, thereby increasing EphA2 phosphorylation and decreasing EphA2 levels. In other embodiments, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and inhibits cancer cell colony formation in soft agar, inhibits tubular network formation in three-dimensional basement membrane or extracellular matrix preparation, preferentially binds to an EphA2 epitope that is exposed on cancer cells but not non-cancer cells, and / or has a low Koff, thereby, inhibiting tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA2 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
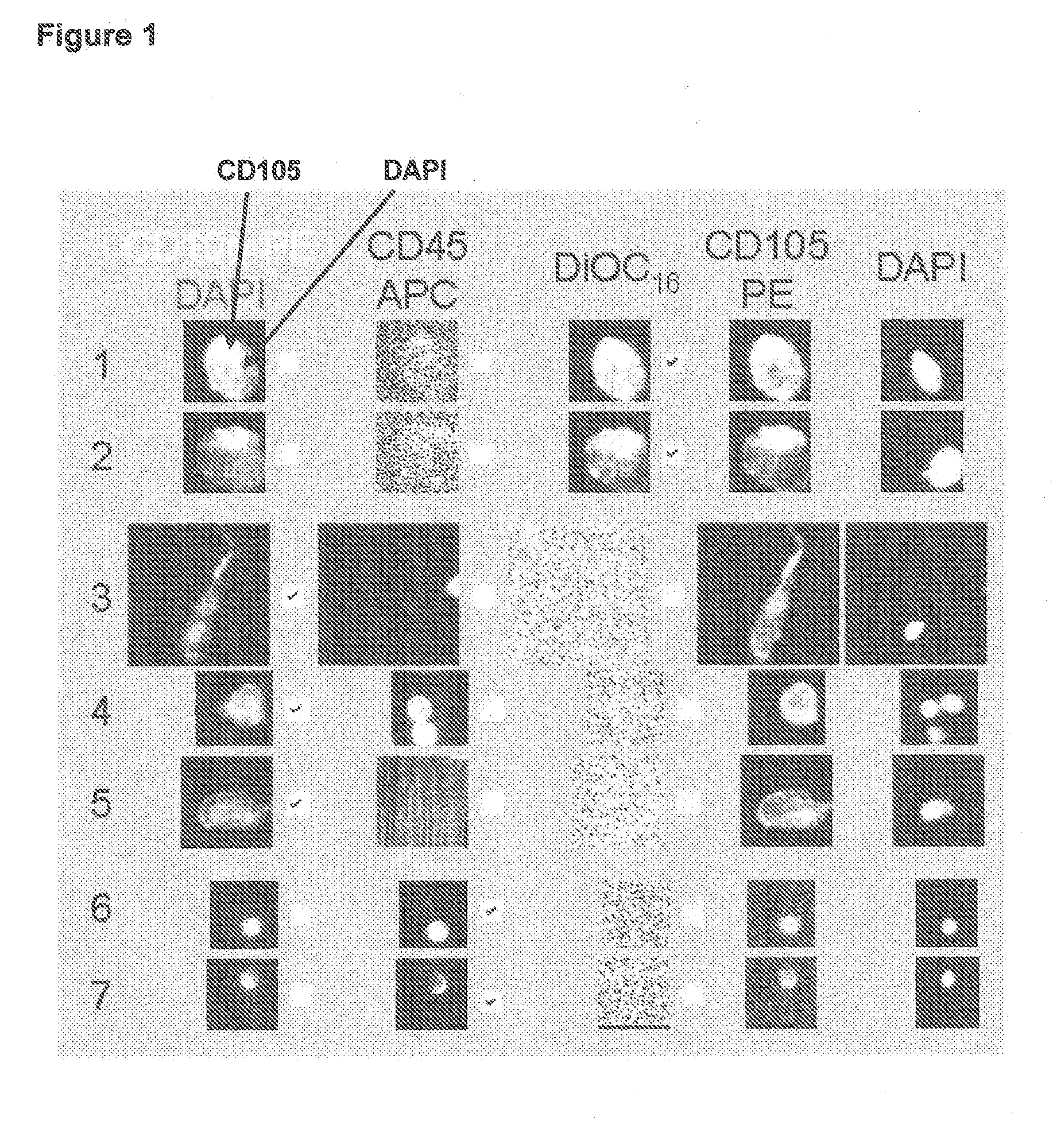
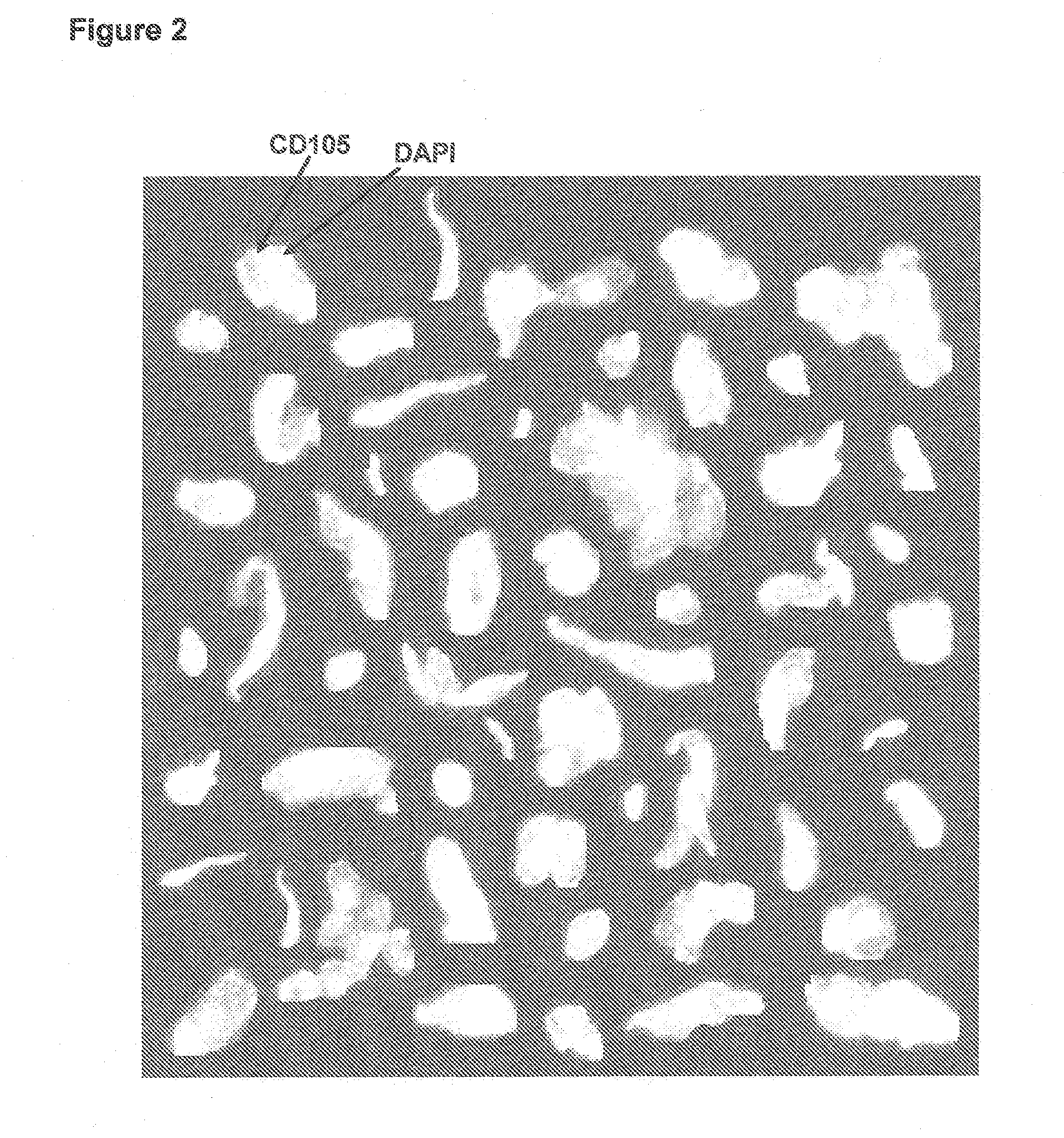
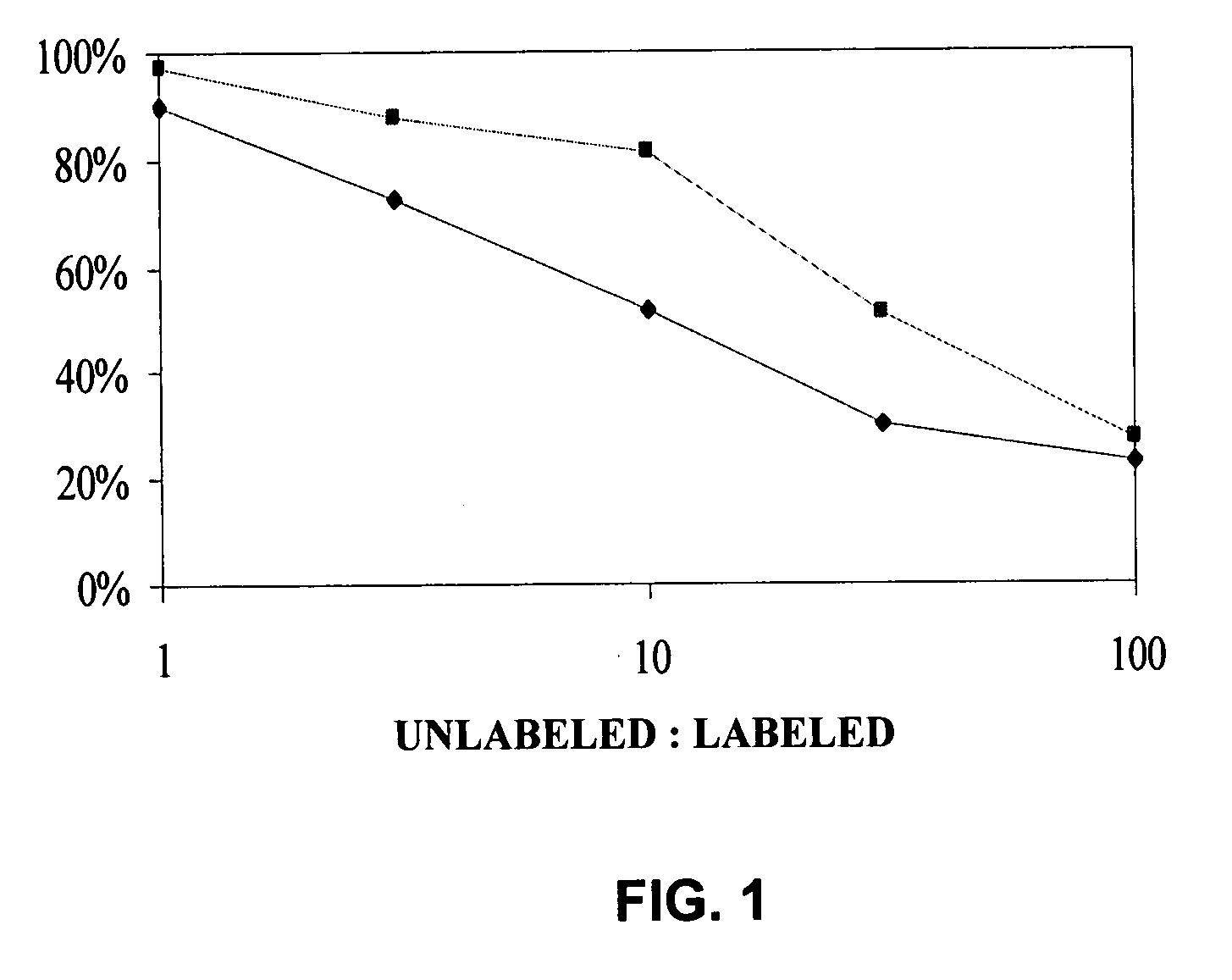
Method of assessing metastatic carcinomas from circulating endothelial cells and disseminated tumor cells
InactiveUS20090191535A1Microbiological testing/measurementDead animal preservationCirculating endothelial cellPrimary breast cancer
A method for assessing cancer in test subjects is described based upon enumeration of circulating endothelial cells and / or disseminated tumor cells in a test subject. This method is used to quantify disseminated tumor cells. Correlations with circulating tumor cells provides prognostic information with high accuracy in assessing the risk of recurrence in patients with primary breast cancer.
Owner:JANSSEN DIAGNOSTICS LLC
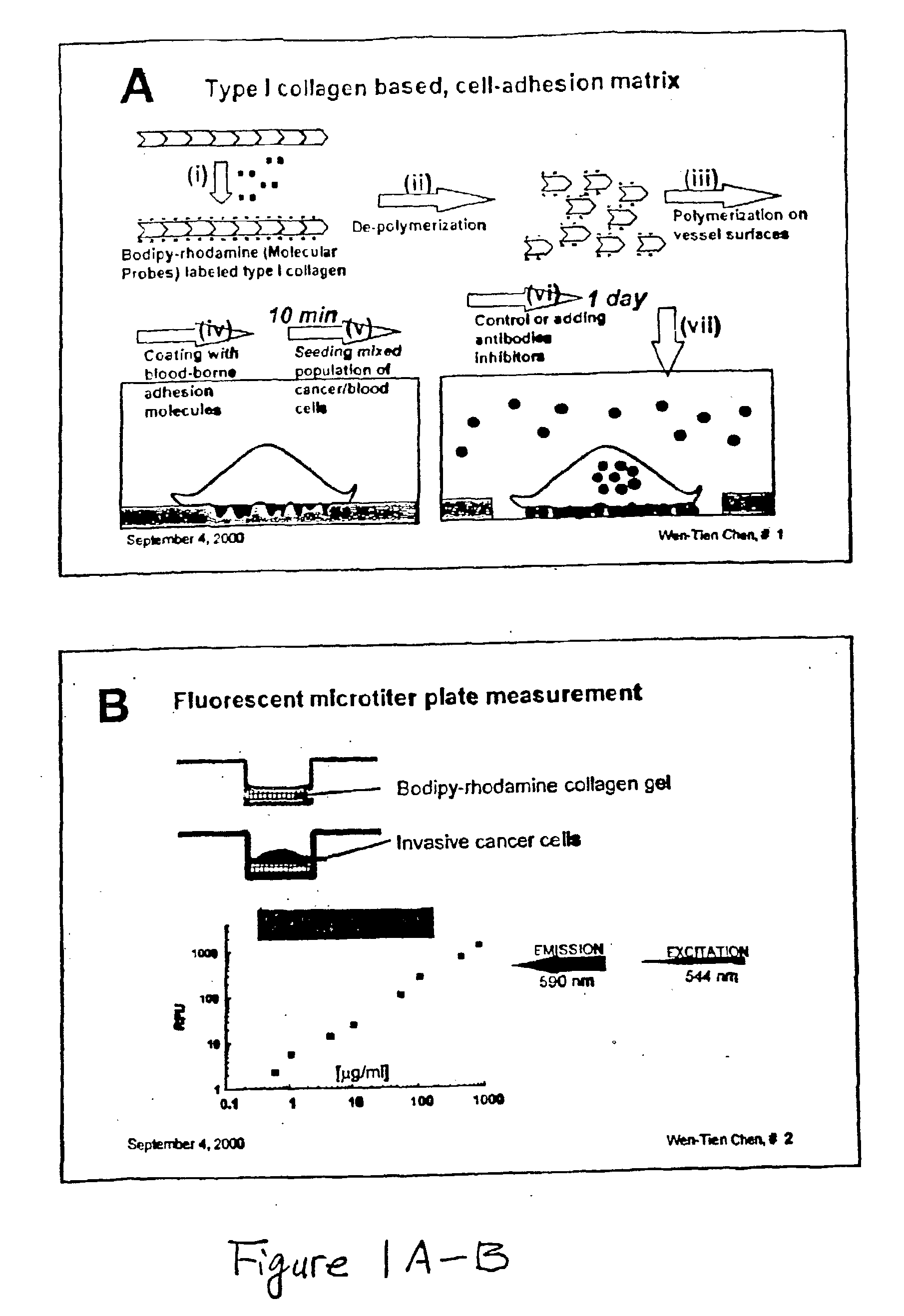
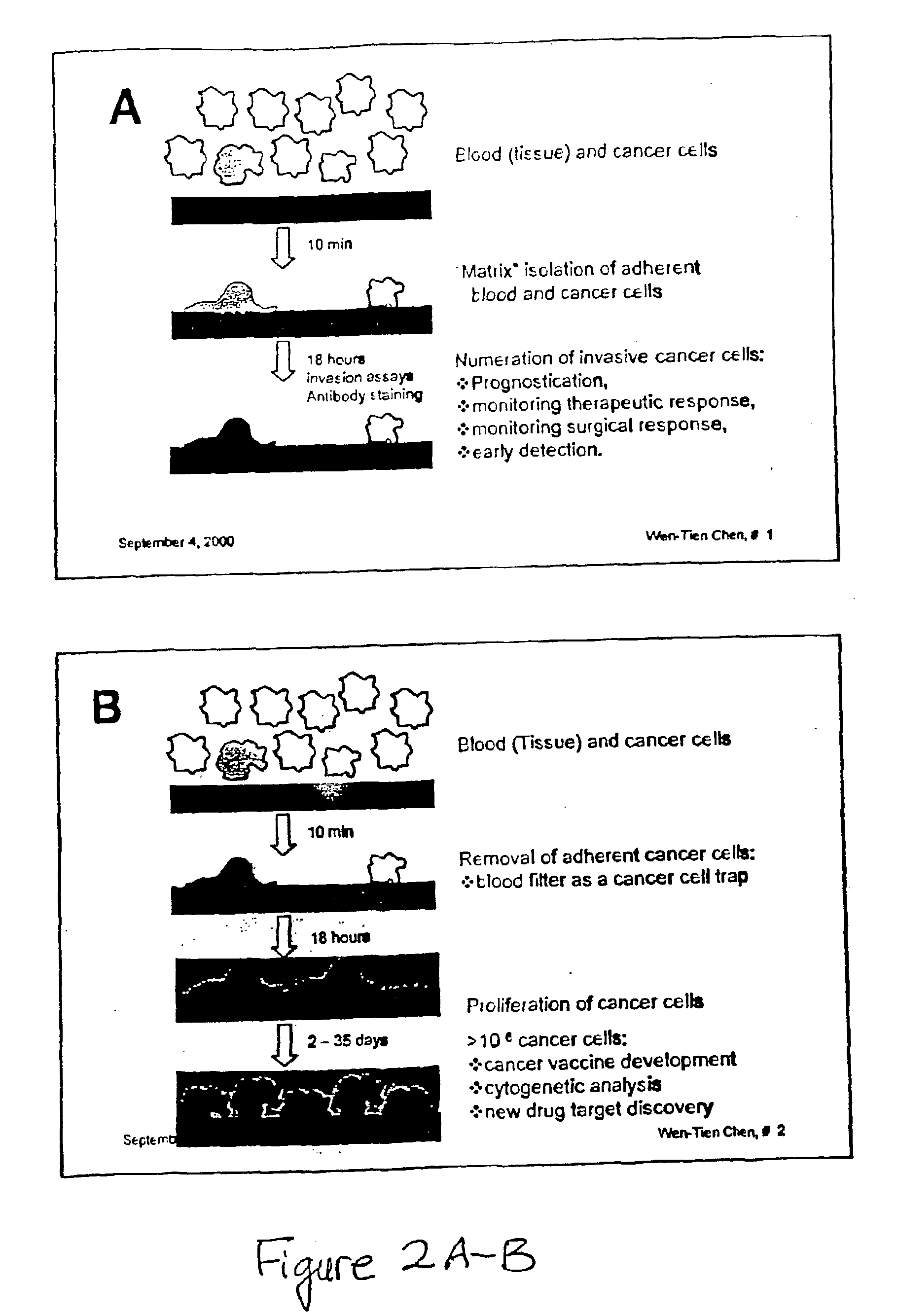
Method and compositions for isolating metastatic cancer cells, and use in measuring metastatic potentatial of a cancer thereof
InactiveUS20030206901A1Quick checkRapid isolationSolvent extractionOther blood circulation devicesCancer cellProteinase activity
The present invention relates to novel methods and compositions for detection and isolation of cancer cells with metastatic potential. The invention further relates to assays for measuring the metastatic potential of such cancer cells and drug screening assays for the identification of agents having anti-metastatic potential. The present invention further provides methods and compositions for inhibiting the metastatic potential of cancer cells by modulating the activity of serine integral membrane proteases [(SIMP) consisting of seprase and dipedidyl peptidase IV (DPPIV)]expressed on the surface of metastasizing cancer cells.
Owner:THE RES FOUND OF STATE UNIV OF NEW YORK
Method of tumor regression with VEGF inhibitors
ActiveUS20040265309A1Peptide/protein ingredientsAntibody mimetics/scaffoldsLymphatic SpreadFactor ii
Methods of regressing or inhibiting a tumor in a subject by administering an agent capable of blocking, inhibiting, or ameliorating vascular endothelial growth factor (VEGF)-mediated activity to a subject in need thereof such that the tumor is regressed or inhibited. The method of the invention results in a reduction of tumor size and inhibition of tumor metastases. This method is particularly useful for patients suffering from bulky, metastatic cancers.
Owner:REGENERON PHARM INC +1
Heterocyclic Compounds For Preventing And Treating Disorders Associated With Excessive Bone Loss
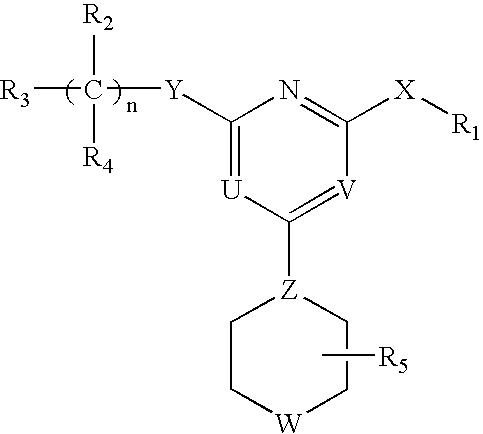
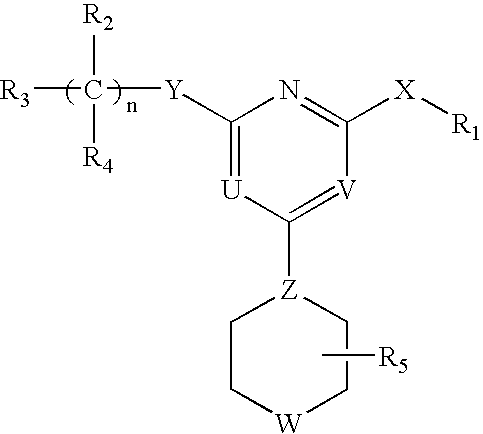
This invention relates to pyrimidine compounds of formula (I), formula (I′), and formula (I″):and pharmaceutically acceptable salts, solvates, clathrates, and prodrugs thereof, wherein R1, R2, R3, R4, R5, U, V, W, X, Y, Z, and n are defined herein. This invention also relates to compositions comprising these compounds and methods for using them. The compounds and compositions of this invention are useful to treat or prevent disorders associated with excessive bone loss, including, without limitation periodontal disease, non-malignant bone disorders (such as osteoporosis, Pagers-disease of bone, osteogenesis imperfecta, fibrous dysplasia, and primary hyperparathyroidism) estrogen deficiency, inflammatory bone loss, bone malignancy, arthritis, osteopetrosis, and certain cancer-related disorders (such as hypercalcemia of malignancy (HCM), osteolytic bone lesions of multiple myeloma and osteolytic bone metastases of breast cancer and other metastatic cancers).
Owner:SYNTA PHARMA CORP
Method for predicting progression free and overall survival at each follow-up time point during therapy of metastatic breast cancer patients using circulating tumor cells
InactiveUS20090061456A1Less side effectsImprove the quality of lifeDisease diagnosisBiological testingOncologyDisease progression
A cancer test having prognostic utility in predicting time to disease progression, overall survival, and response to therapy in patients with MBC based upon the presence and number of CTC's. The Cell Spotter® System is used to enumerate CTC's in blood. The system immunomagnetically concentrates epithelial cells, fluorescently labels the cells and identifies and quantifies CTC's. The absolute number of CTC's detected in the peripheral blood tumor load is, in part, a factor in prediction of survival, time to progression, and response to therapy. The mean time to survival of patients depended upon a threshold number of 5 CTC's per 7.5 ml of blood. Detection of CTC's in metastatic cancer represents a novel prognostic factor in patients with metastatic cancers, suggests a biological role for the presence of tumor cells in the blood, and indicates that the detection of CTC's could be considered an appropriate surrogate marker for prospective therapeutic clinical trials.
Owner:VERIDEX LCC
Therapeutic peptides for the treatment of metastatic cancer
InactiveUS20060293234A1Invasiveness is thereby reduced and retardedVirusesPeptide/protein ingredientsLymphatic SpreadBinding site
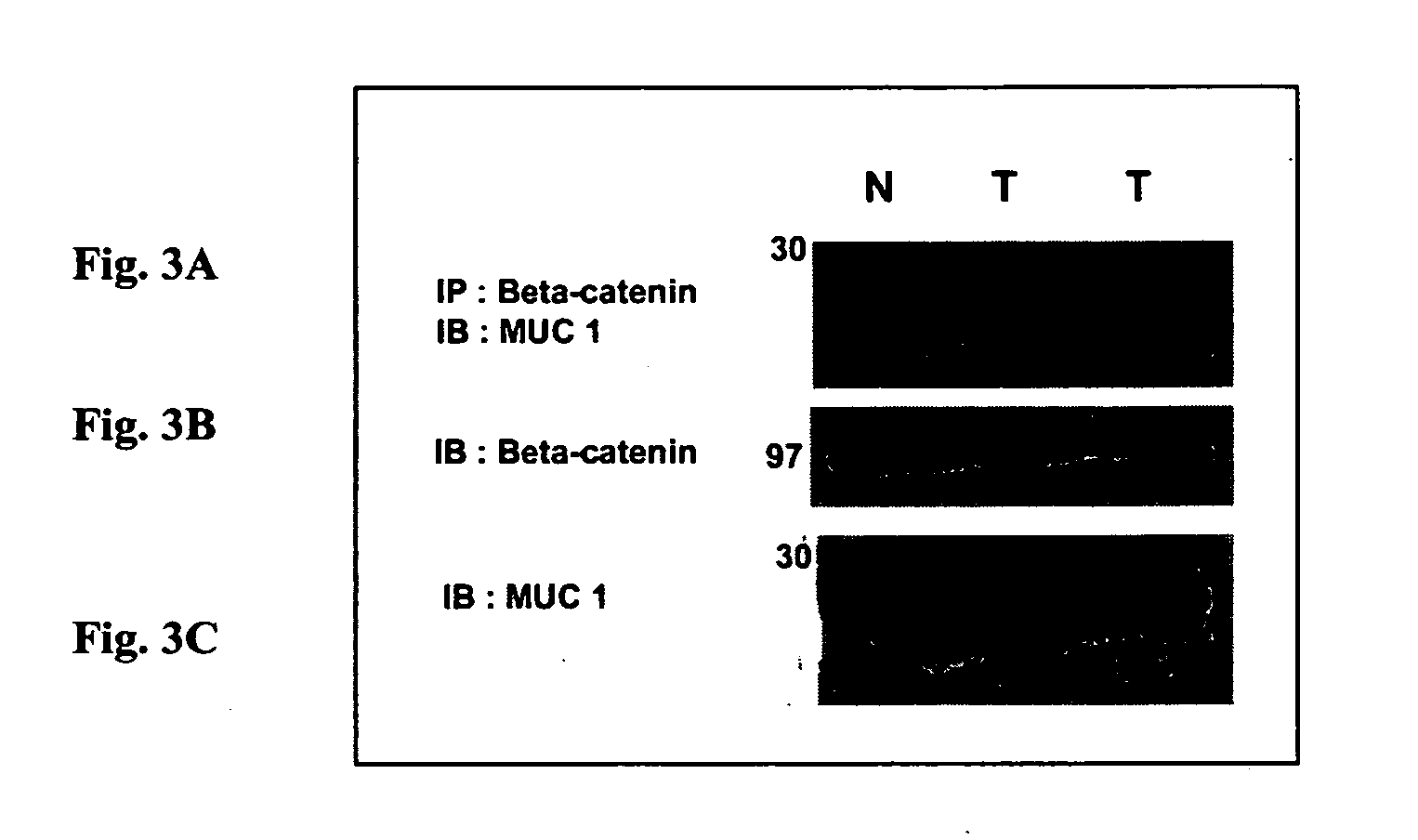
Interaction between MUC1 and β-catenin can be interrupted using polypeptides or antibodies that specifically bind to the binding site on MUC1. Interruption provides the beneficial effect of inhibiting, reducing, and / or retarding invasiveness and metastasis. Fusion polypeptides and antibodies are provided to achieve a therapeutic effect.
Owner:ARIZONA CANCER THERAPEUTICS
Monoclonal antibodies for treatment of cancer
InactiveUS8946388B2Strong formationStrong proliferationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseAntiendomysial antibodies
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
Method for the diagnosis, prognosis and treatment of cancer metastasis
InactiveUS20170002357A1Peptide/protein ingredientsMicrobiological testing/measurementCancer preventionLymphatic Spread
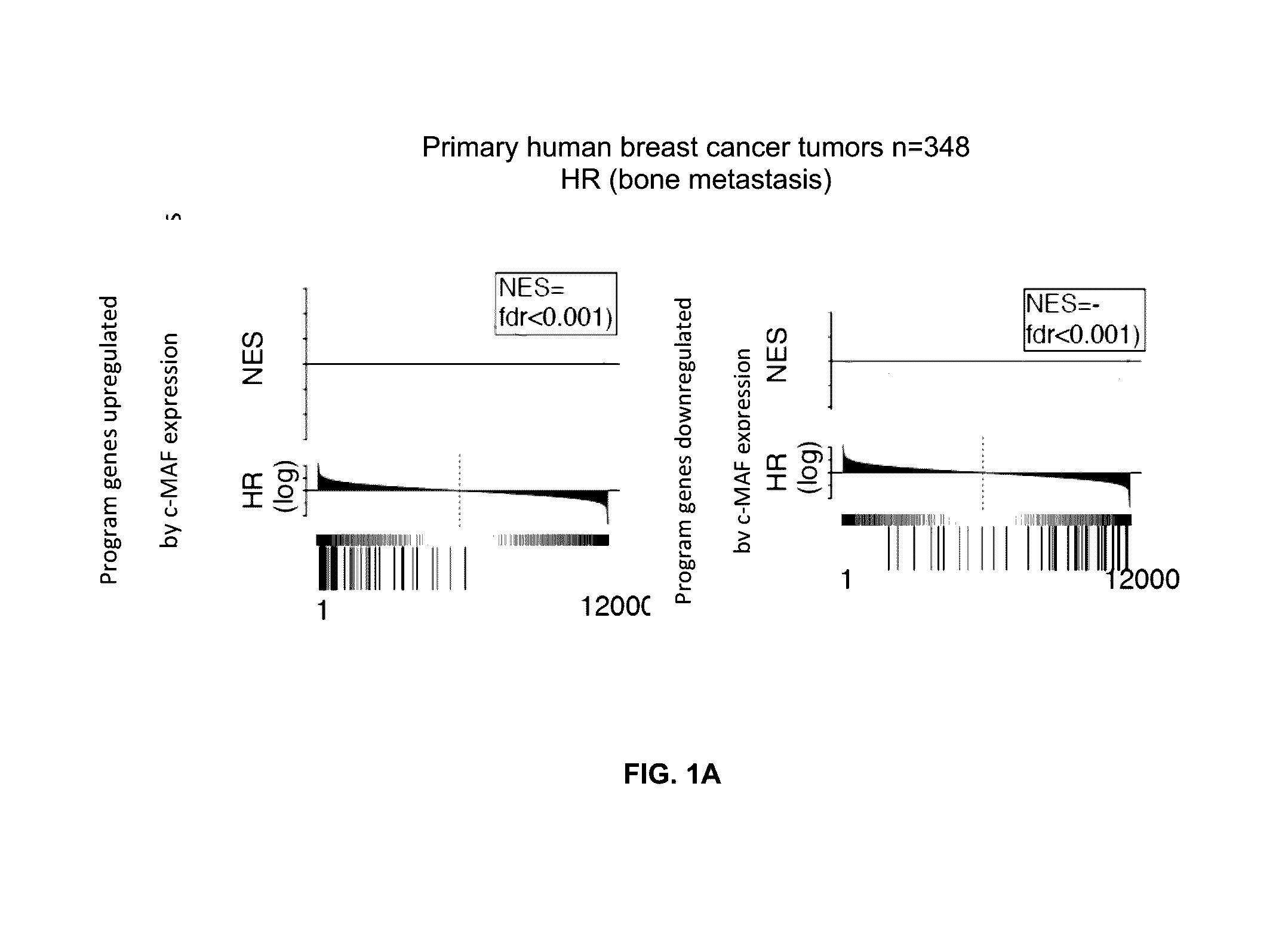
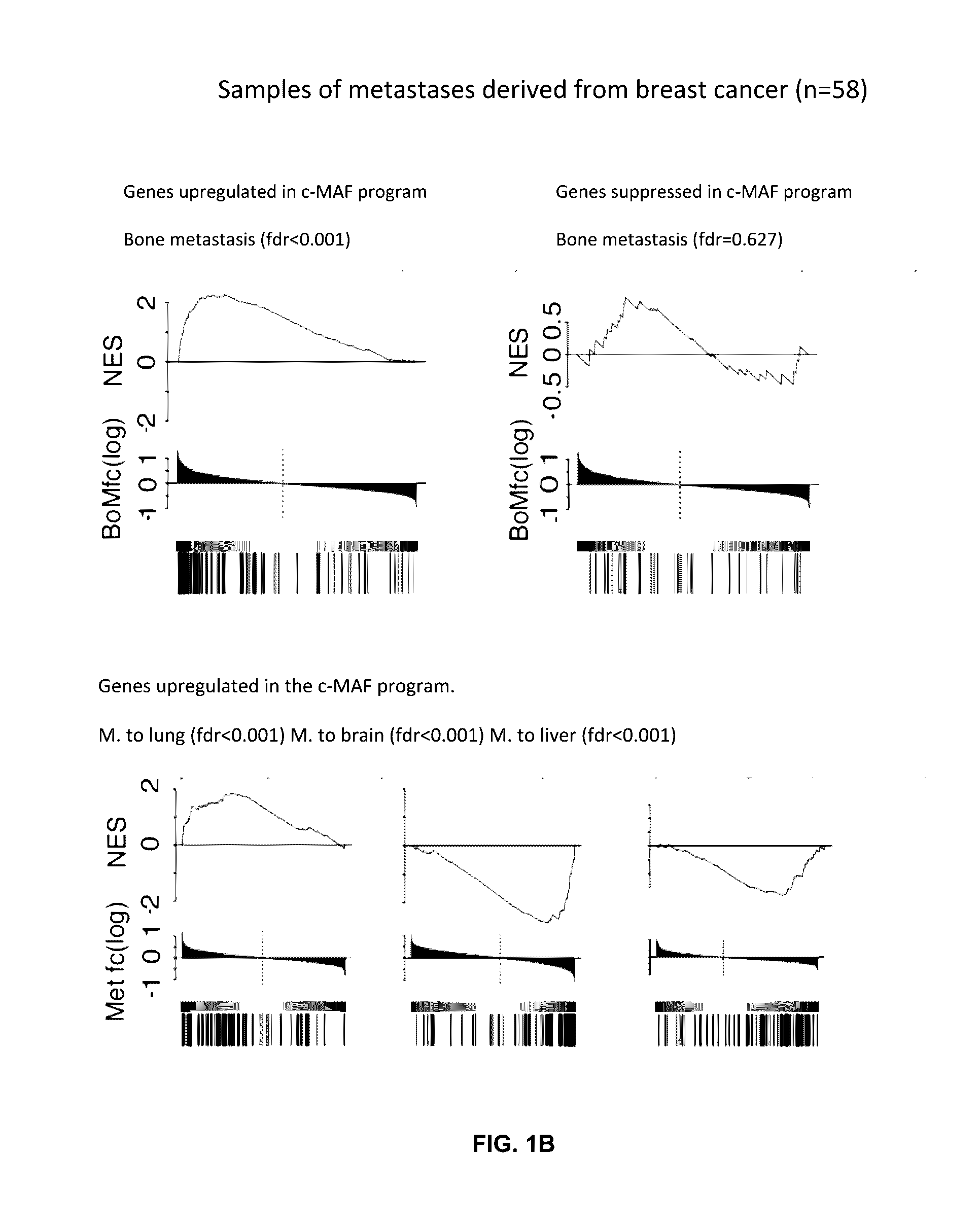
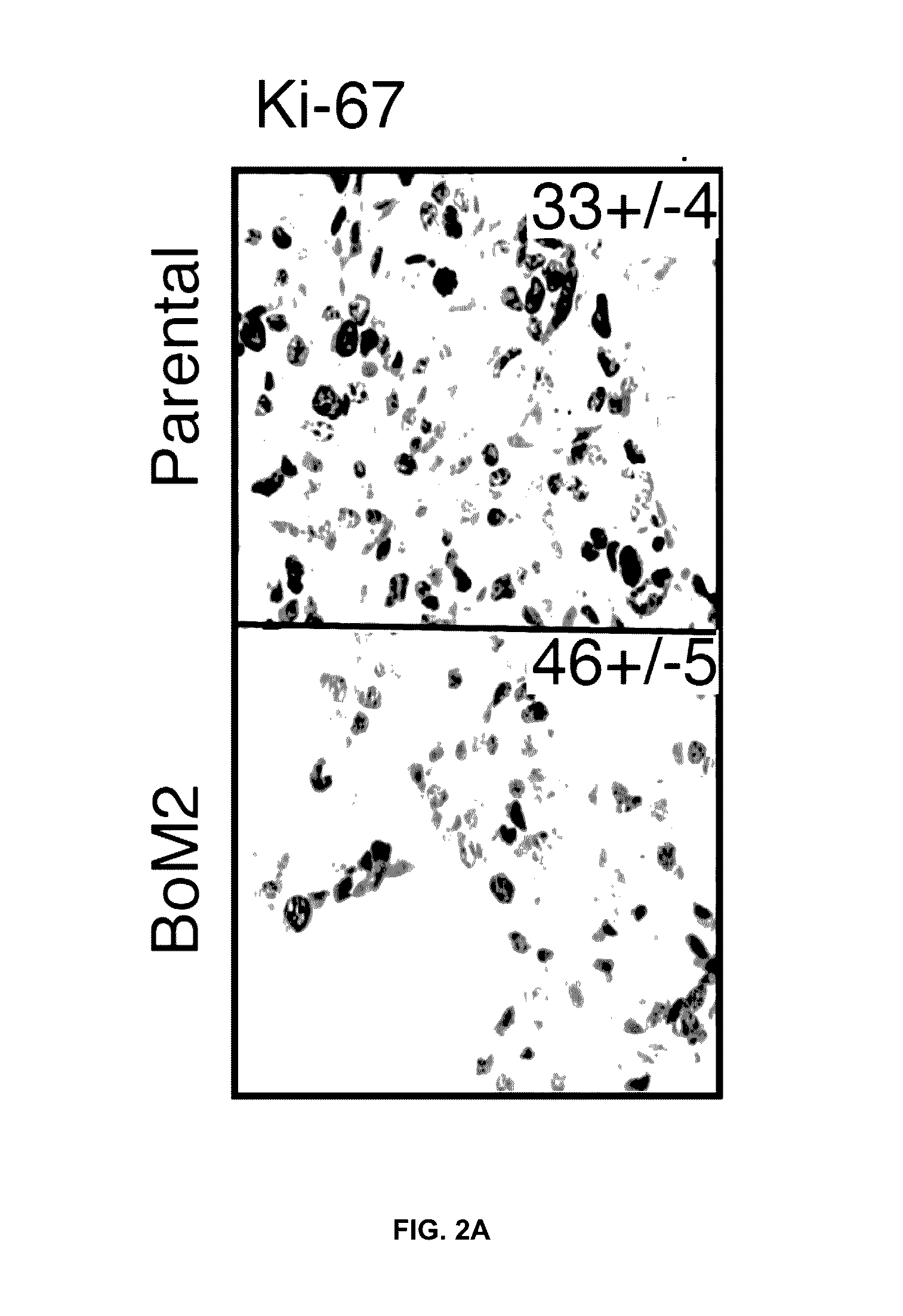
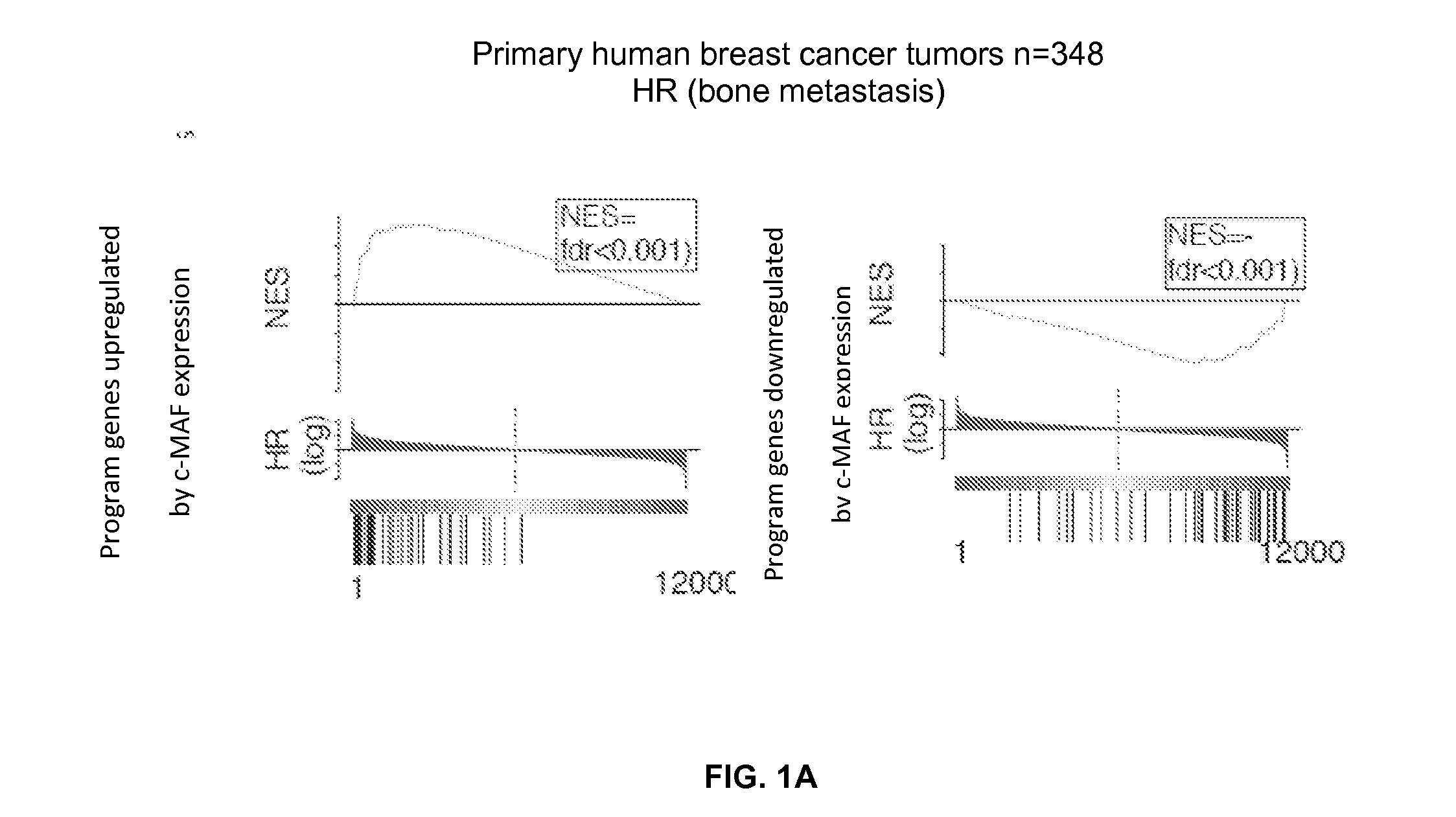
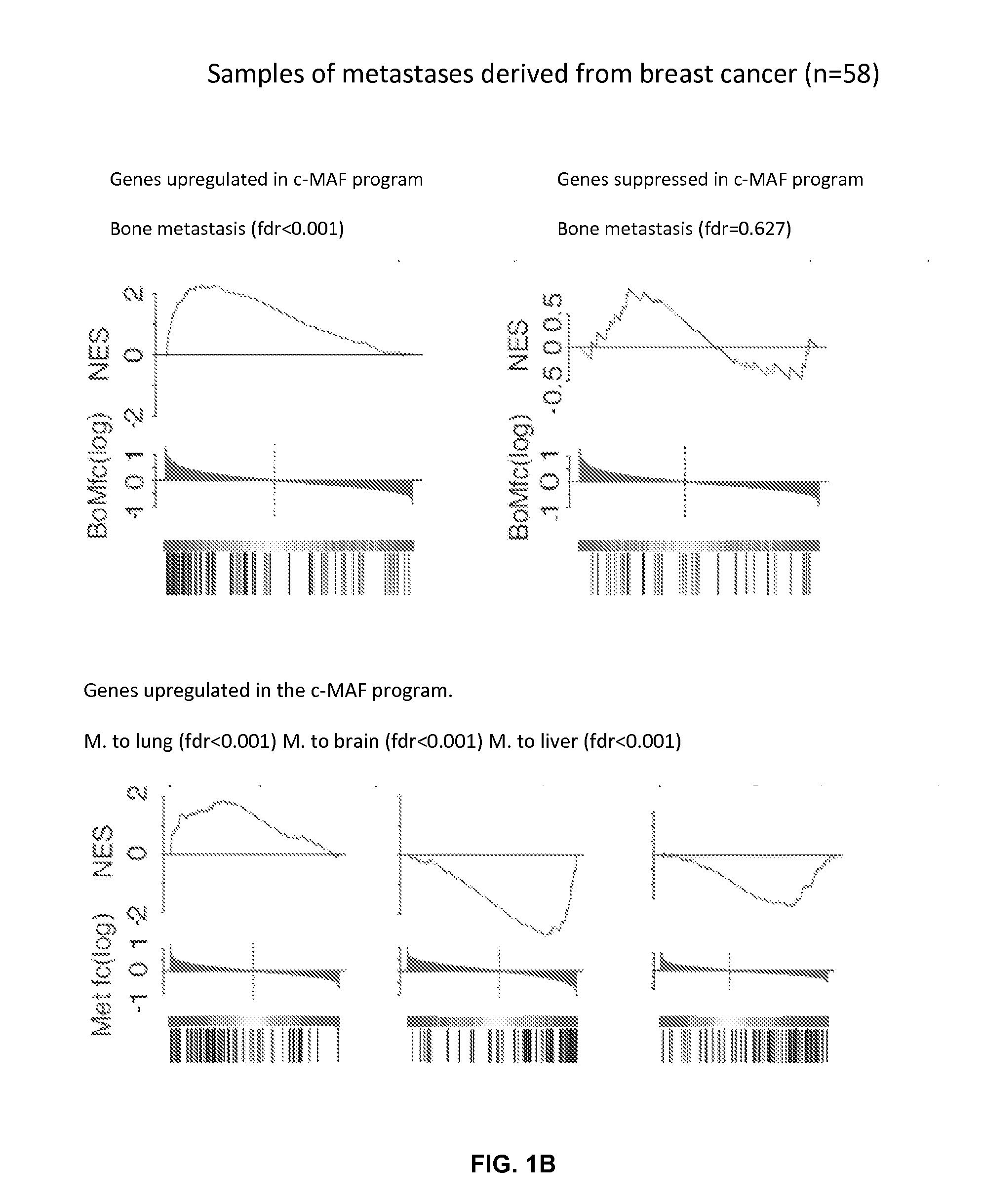
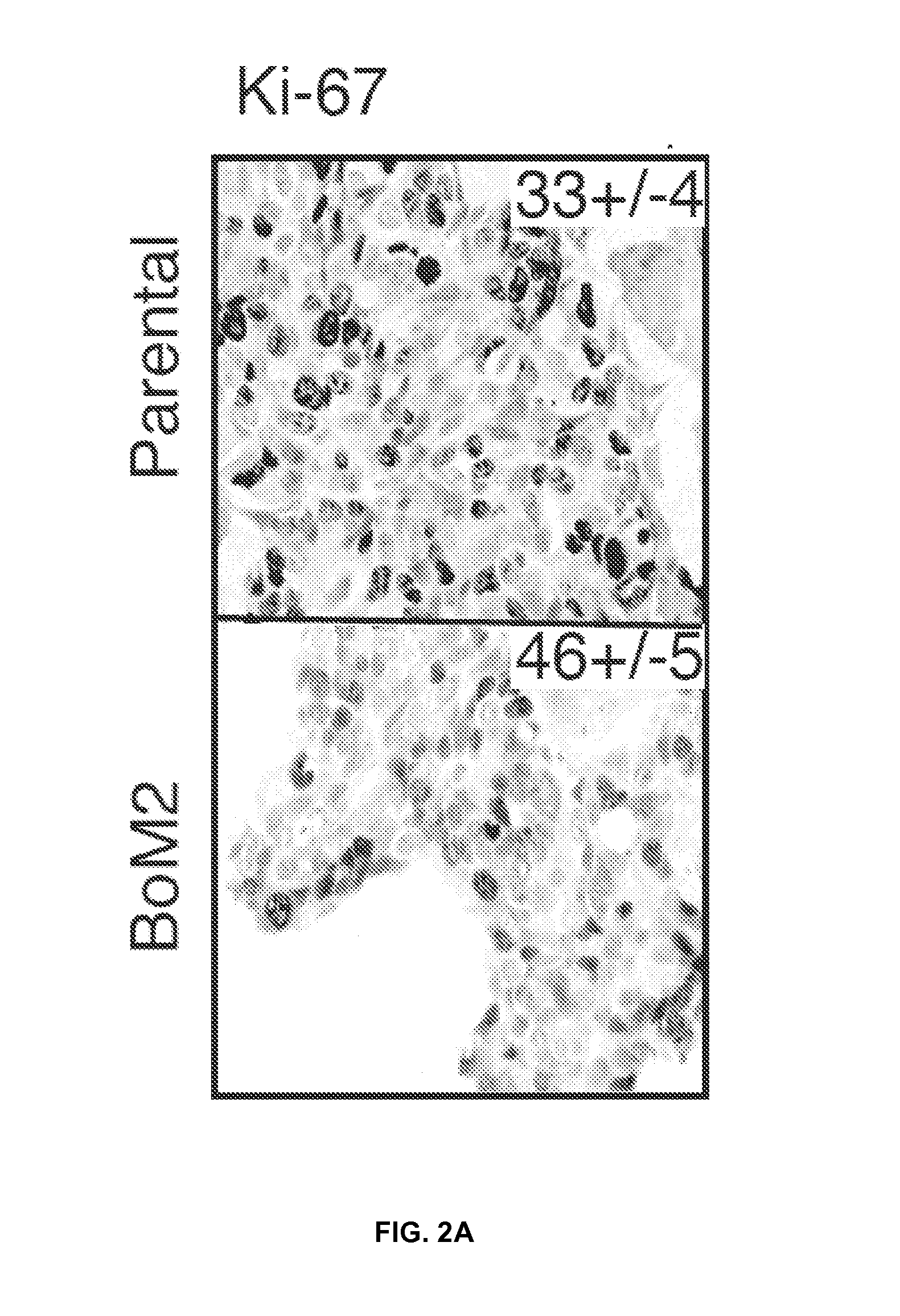
This study describes a method to determine the likelihood of the development of metastasis in a subject suffering from cancer, in addition to a method to design a customized therapy in a subject suffering from cancer, in particular breast, colon, lung, kidney and thyroid cancer, based on the determination of the expression level of one or more genes whose expression is modulated by an increase in c-MAF expression. It also describes a method for the identification of marker genes with a propensity for metastatic cancer based on inducing the modulation of the c-MAF expression Finally, the use of PTHLH and PODXL inhibitors and RERG activators in the treatment and / or prevention of the cancer, in particular breast, colon, lung, kidney and thyroid cancer.
Owner:FUNDACIO INST DE RECERCA BIOMEDICA (IRB BARCELONA) +1
Method for the diagnosis, prognosis, and tratment of cancer metastasis
InactiveUS20160040247A1Sugar derivativesPeptide/protein ingredientsCancer preventionLymphatic Spread
This study describes a method to determine the likelihood of the development of metastasis in a subject suffering from cancer, in addition to a method to design a customized therapy in a subject suffering from cancer, in particular breast, colon, lung, kidney and thyroid cancer, based on the determination of the expression level of one or more genes whose expression is modulated by an increase in c-MAF expression. It also describes a method for the identification of marker genes with a propensity for metastatic cancer based on inducing the modulation of the c-MAF expression Finally, the use of PTHLH and PODXL inhibitors and RERG activators in the treatment and / or prevention of the cancer, in particular breast, colon, lung, kidney and thyroid cancer.
Owner:FUNDACIO INST DE RECERCA BIOMEDICA (IRB BARCELONA) +1
Monoclonal antibodies for treatment of cancer
InactiveUS20110223182A1Minimize adverse effectsStrong formationImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsDiseaseMelanoma
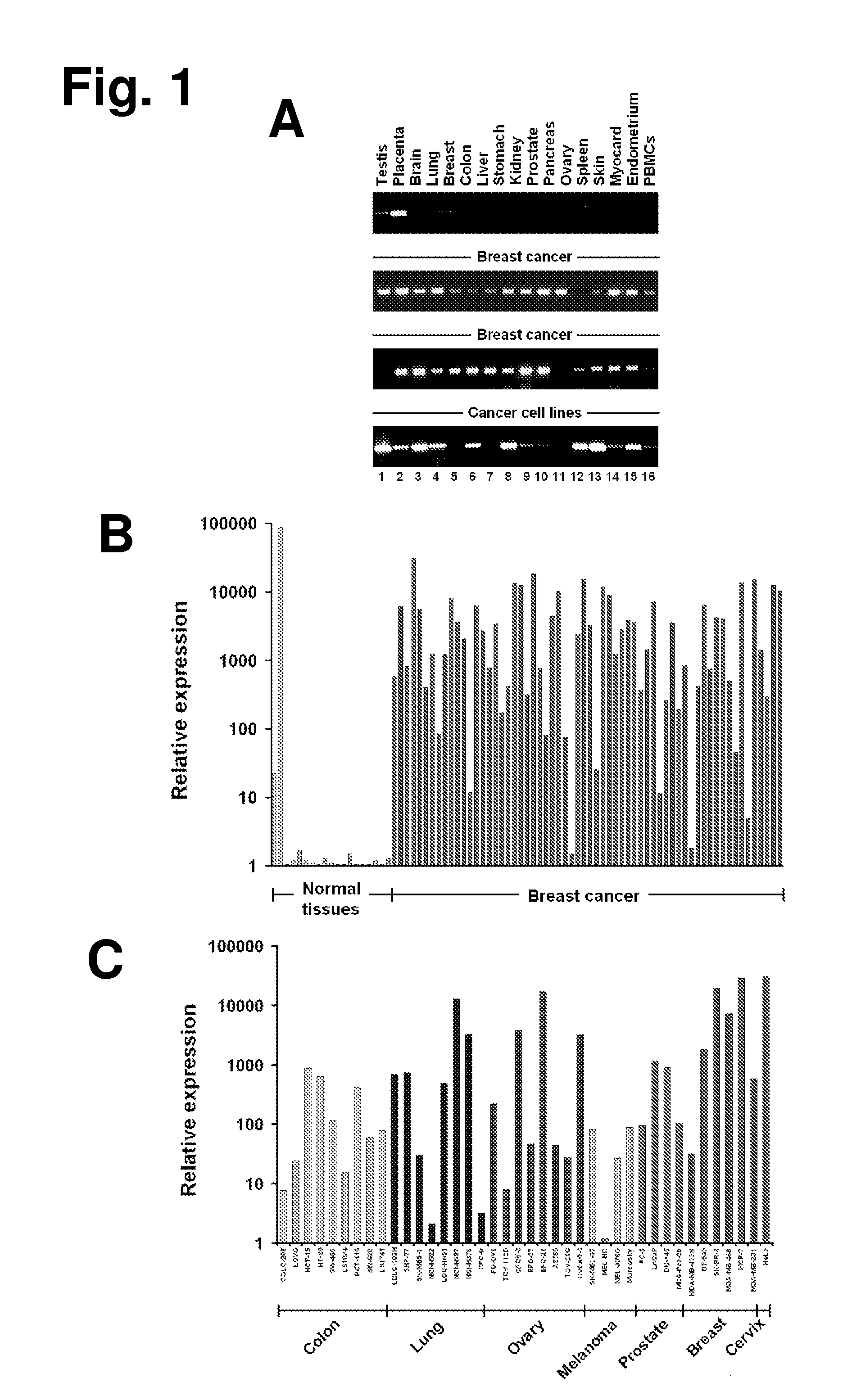
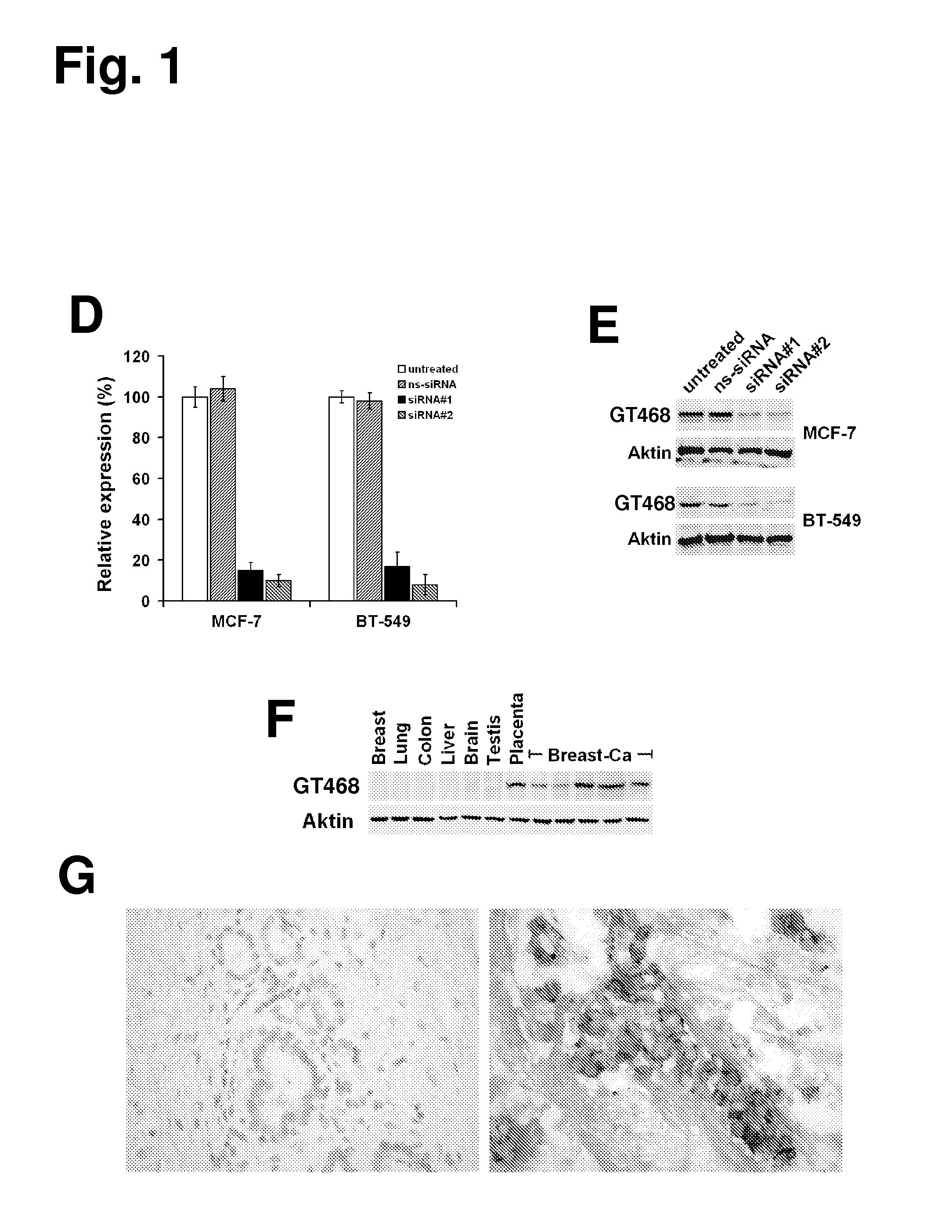
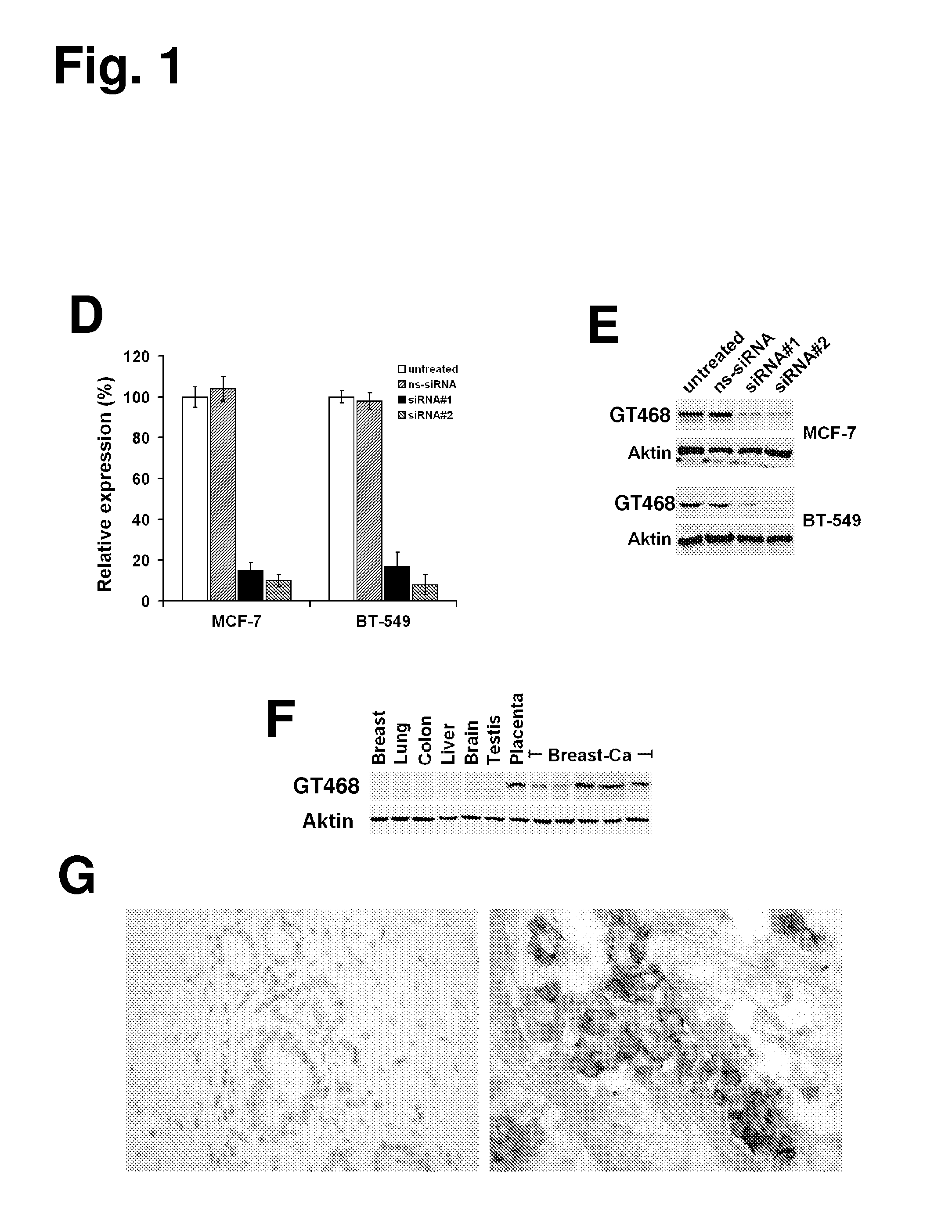
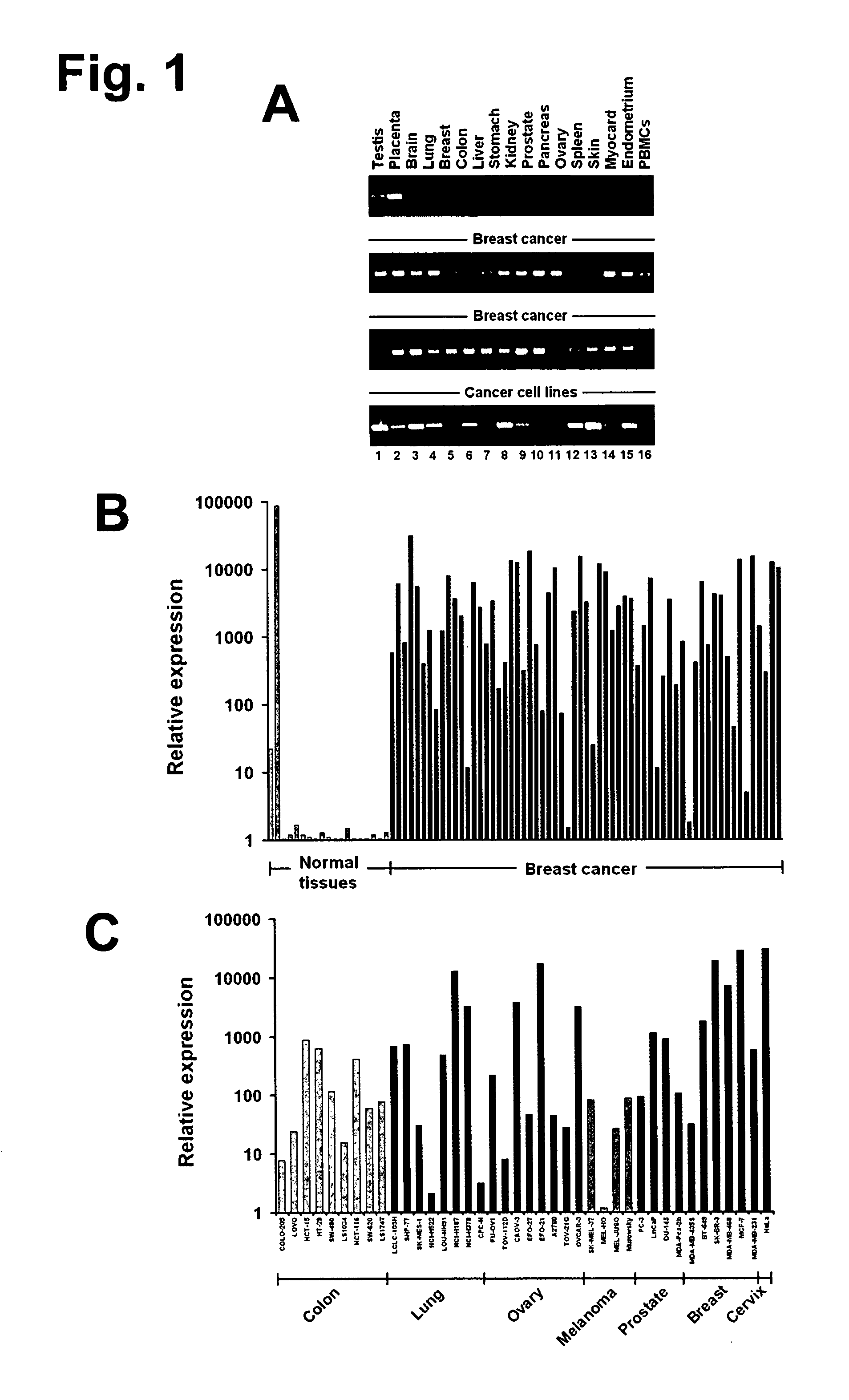
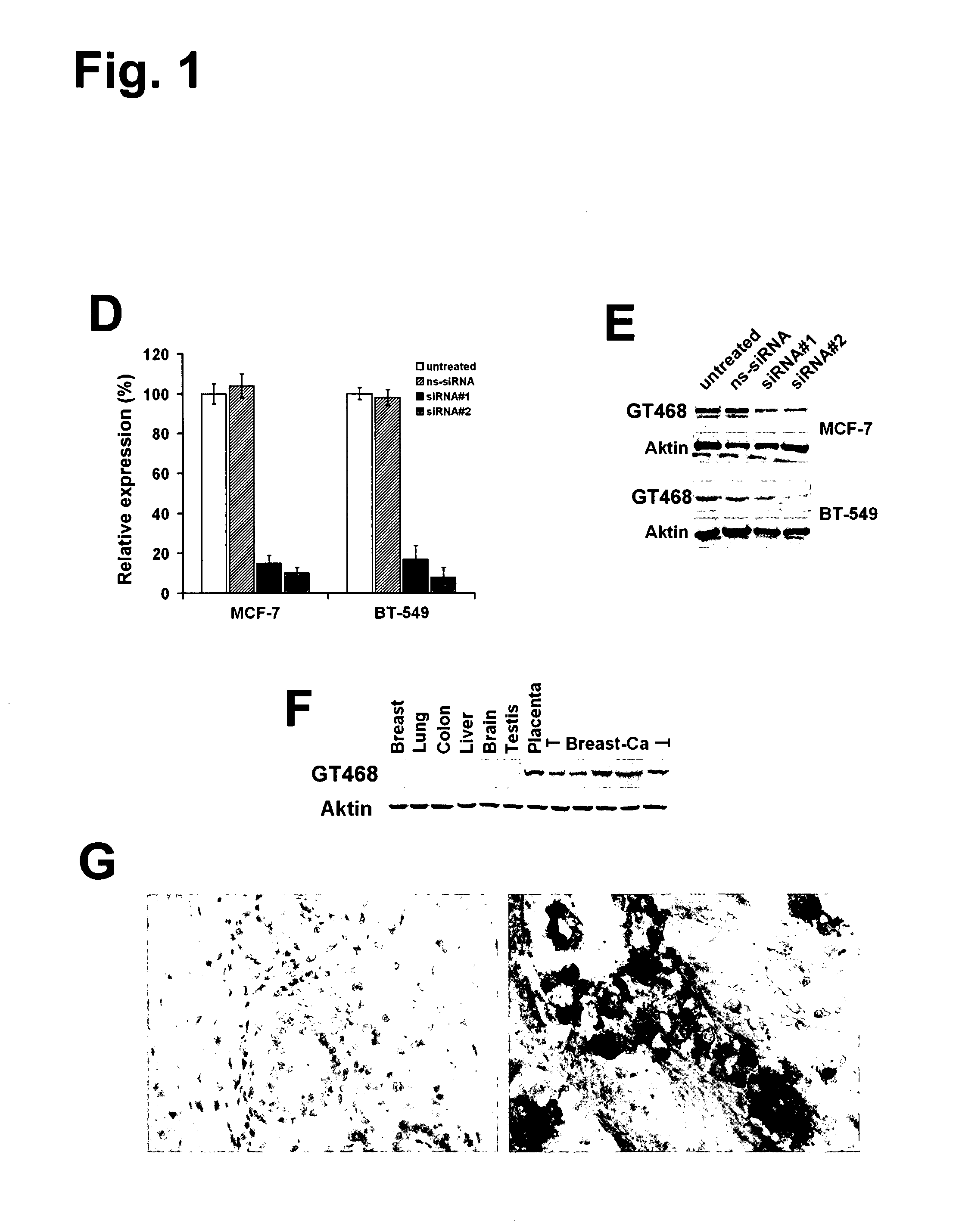
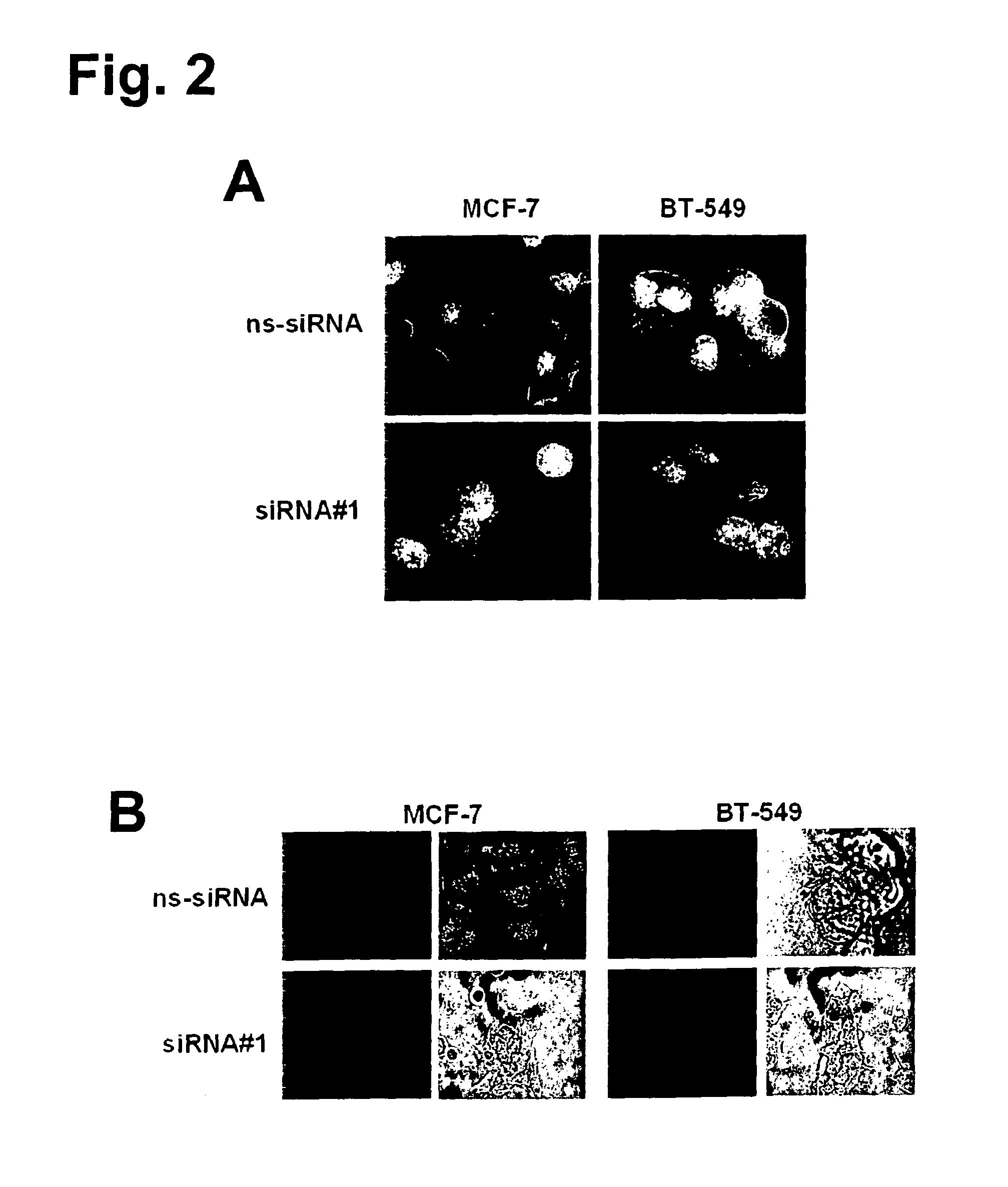
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast Cancer, lung Cancer, gastric Cancer, ovarian Cancer, hepatocellular Cancer, colon Cancer, pancreatic Cancer, esophageal Cancer, head & neck Cancer, kidney Cancer, in particular renal cell Carcinoma, prostate Cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid Cancer, and the metastatic forms thereof. In one embodiment, the rumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
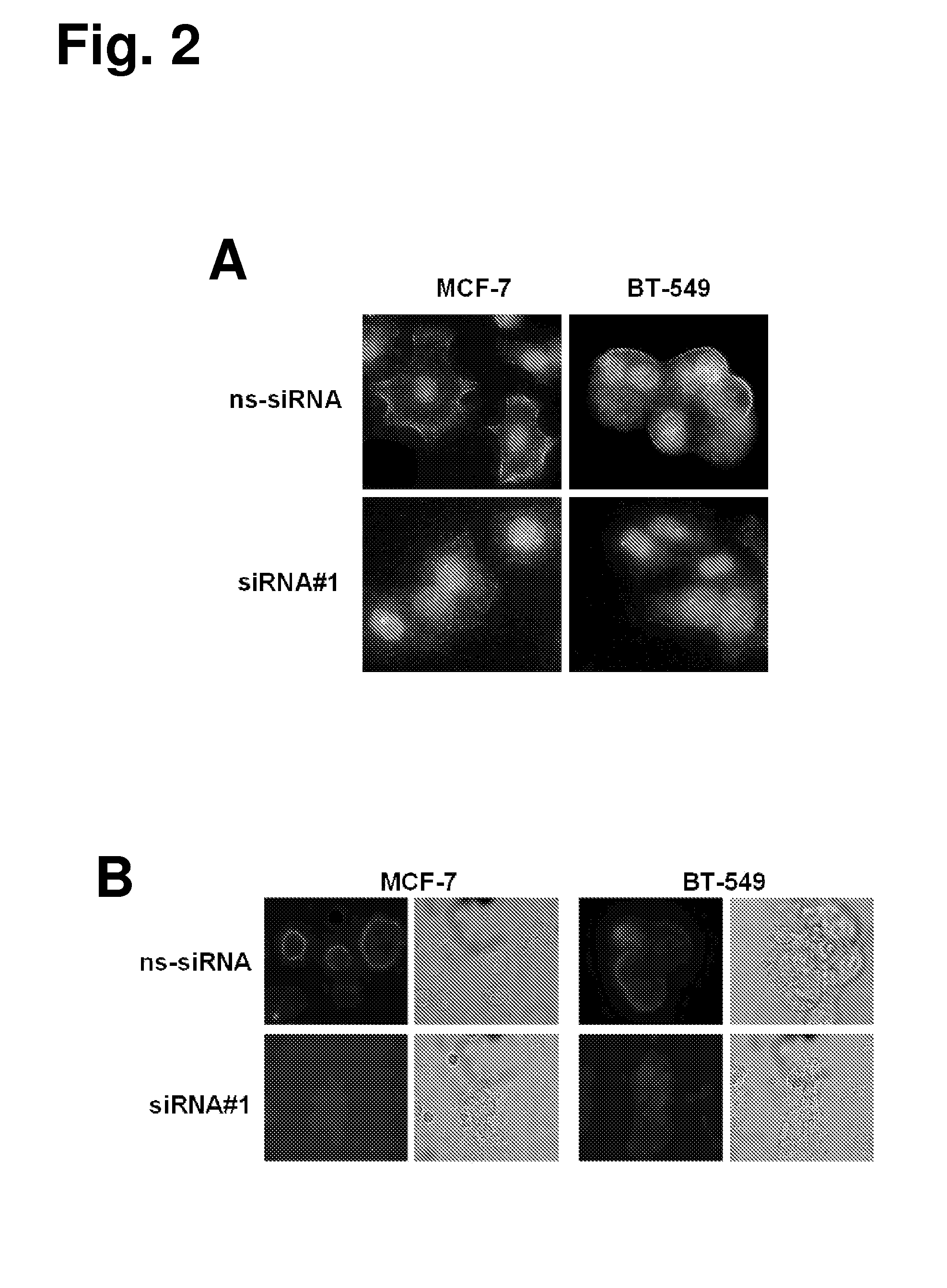
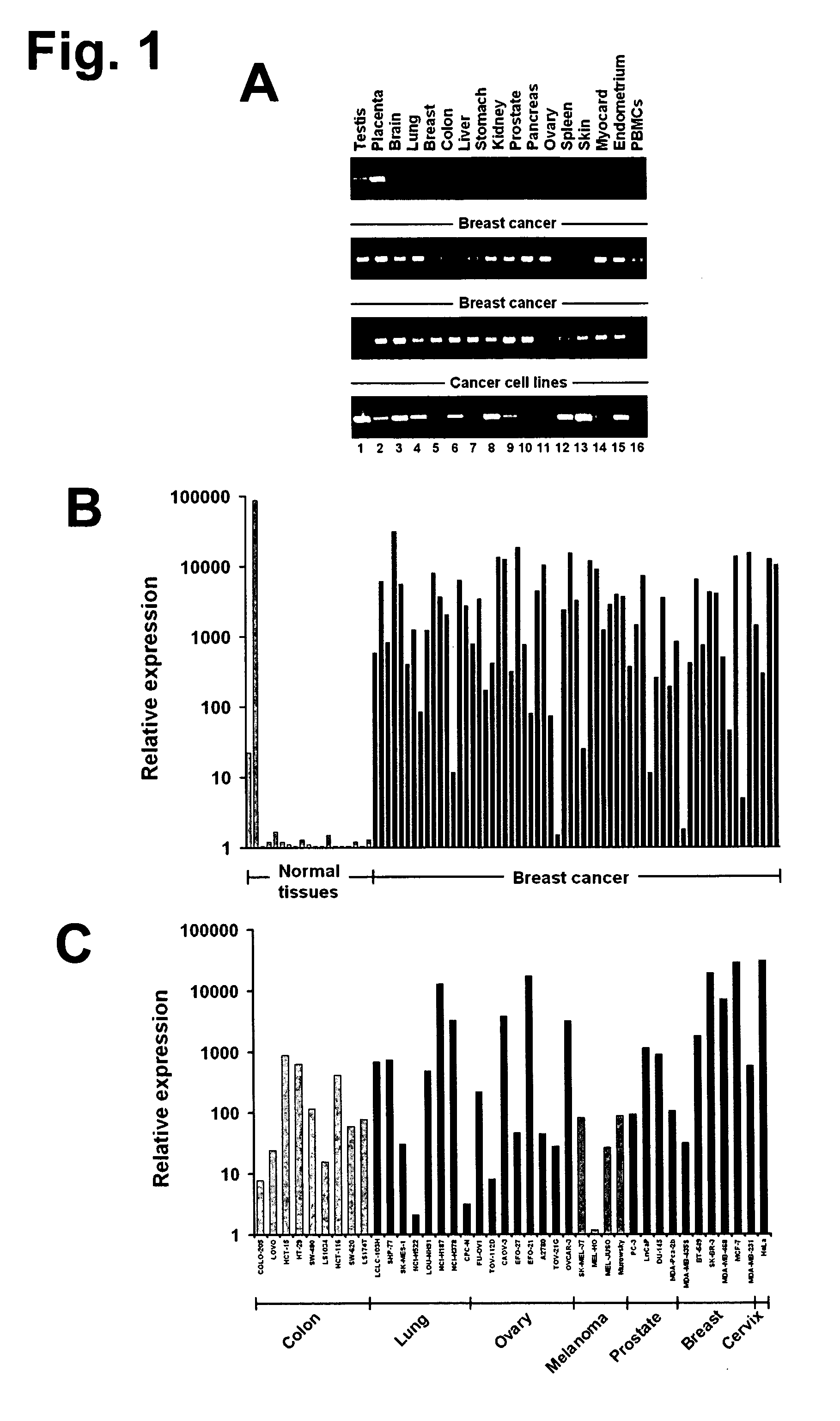
Transcriptome deconvolution of metastatic tissue samples
PendingUS20200210852A1Improve responseIncrease resistanceMathematical modelsKernel methodsTissue sampleExpression gene
A platform for transcriptome deconvolution of gene expression data is provided and may be used in assessing metastatic cancer samples. The deconvolution is performed using an unsupervised clustering technique, such as grade of membership, that allows for samples to be assigned to multiple clusters during a training process. A deconvolution gene expression model is generated as a result and is used for accurate assess of metastases in subsequent samples.
Owner:TEMPUS LABS INC
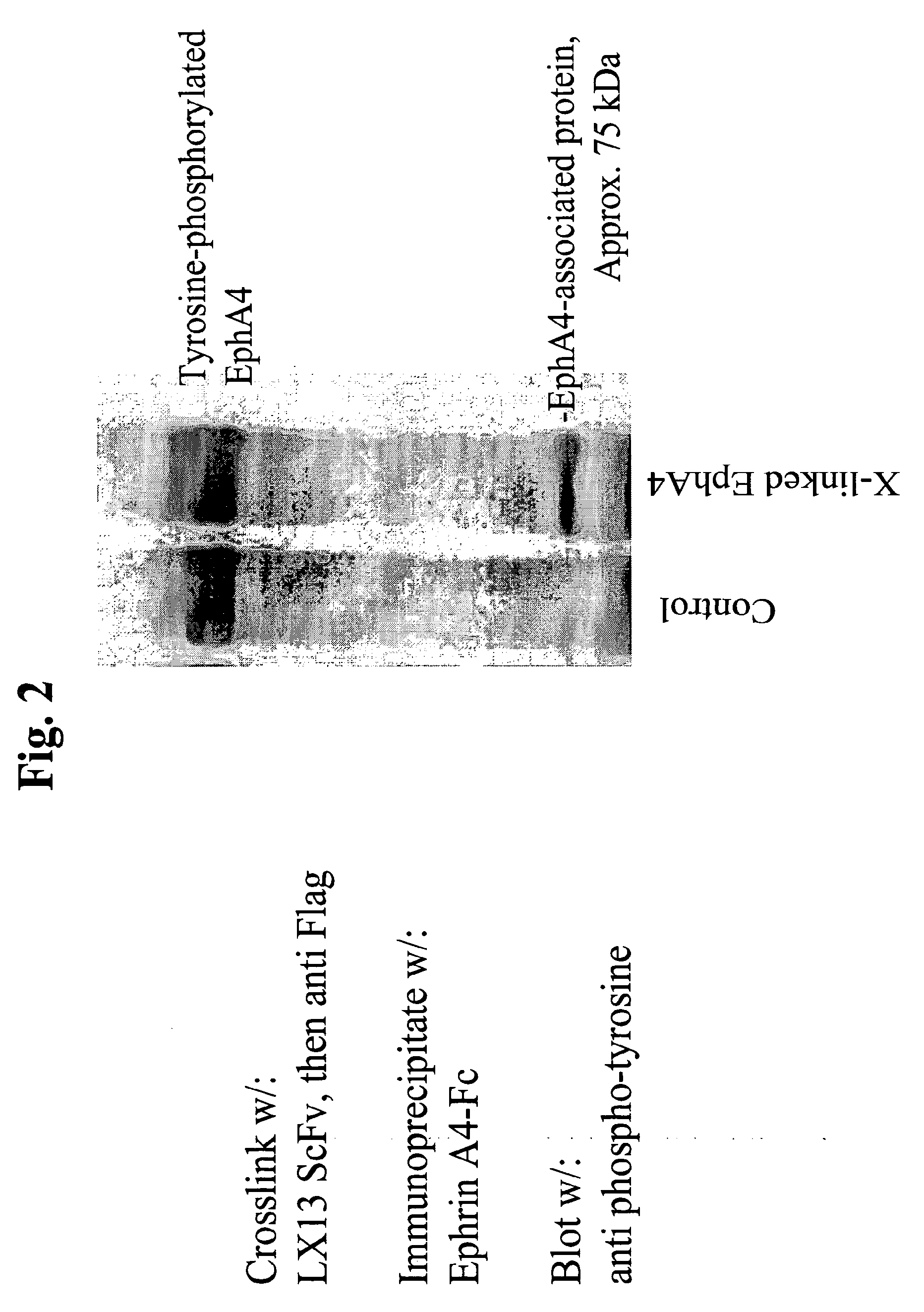
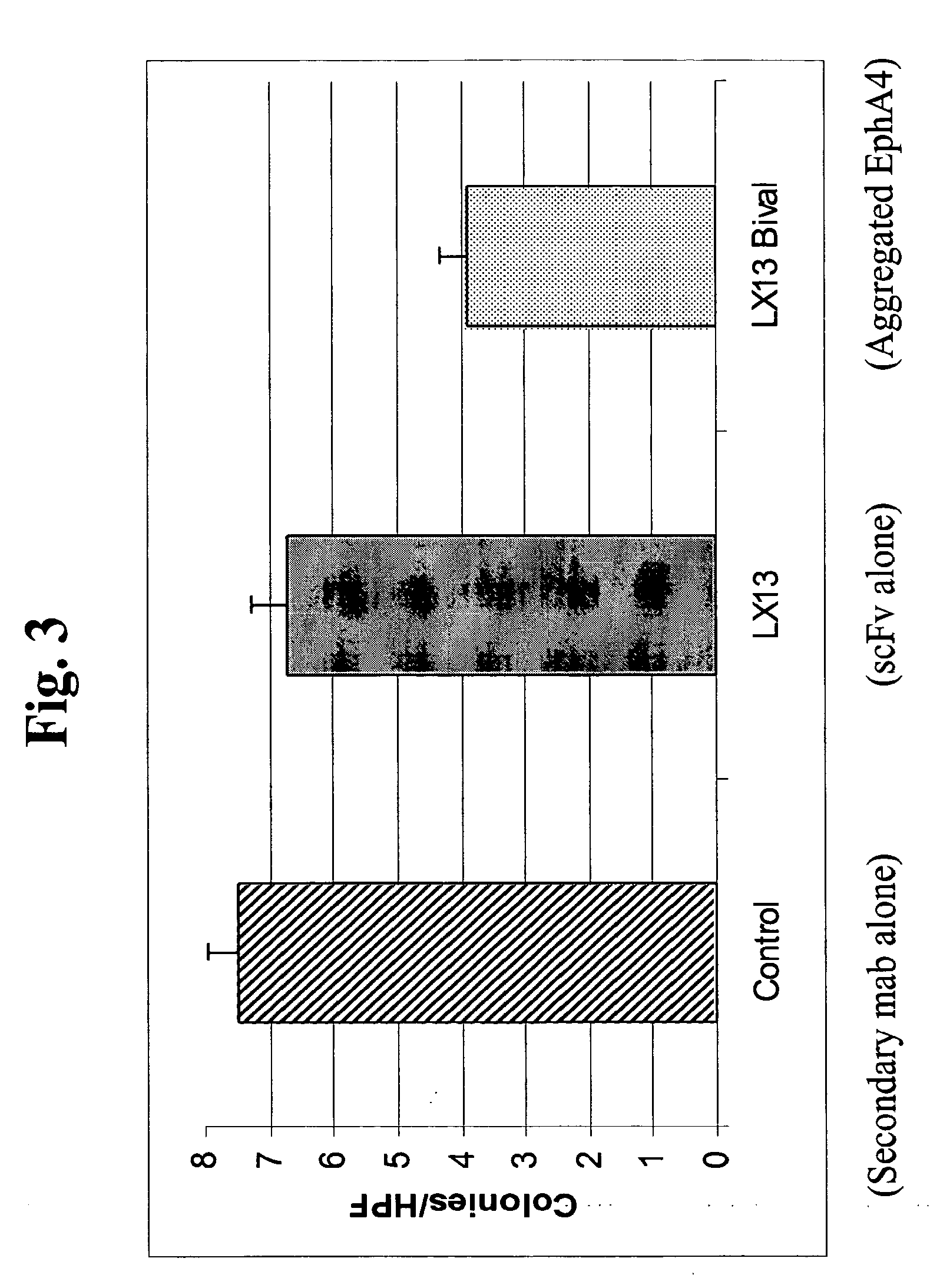
USE OF EphA4 AND MODULATOR OF EphA4 FOR DIAGNOSIS, TREATMENT AND PREVENTION OF CANCER
InactiveUS20100166657A1Promote growthReduce expressionAntibody mimetics/scaffoldsImmunoglobulins against cytokines/lymphokines/interferonsCancer preventionCancer cell
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 and agonize EphA4. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 and inhibit cancer cell colony formation in soft agar or tubular network formation in three-dimensional basement membrane or extracellular matrix preparation. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that preferentially binds to an EphA4 epitope that is exposed on cancer cells but not non-cancer cells. In another embodiment, the methods of the invention comprise the administration of an effective amount of one or more antibodies that bind to EphA4 with a very low Koff to reduce EphA4 expression and, thereby, inhibit tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA4 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
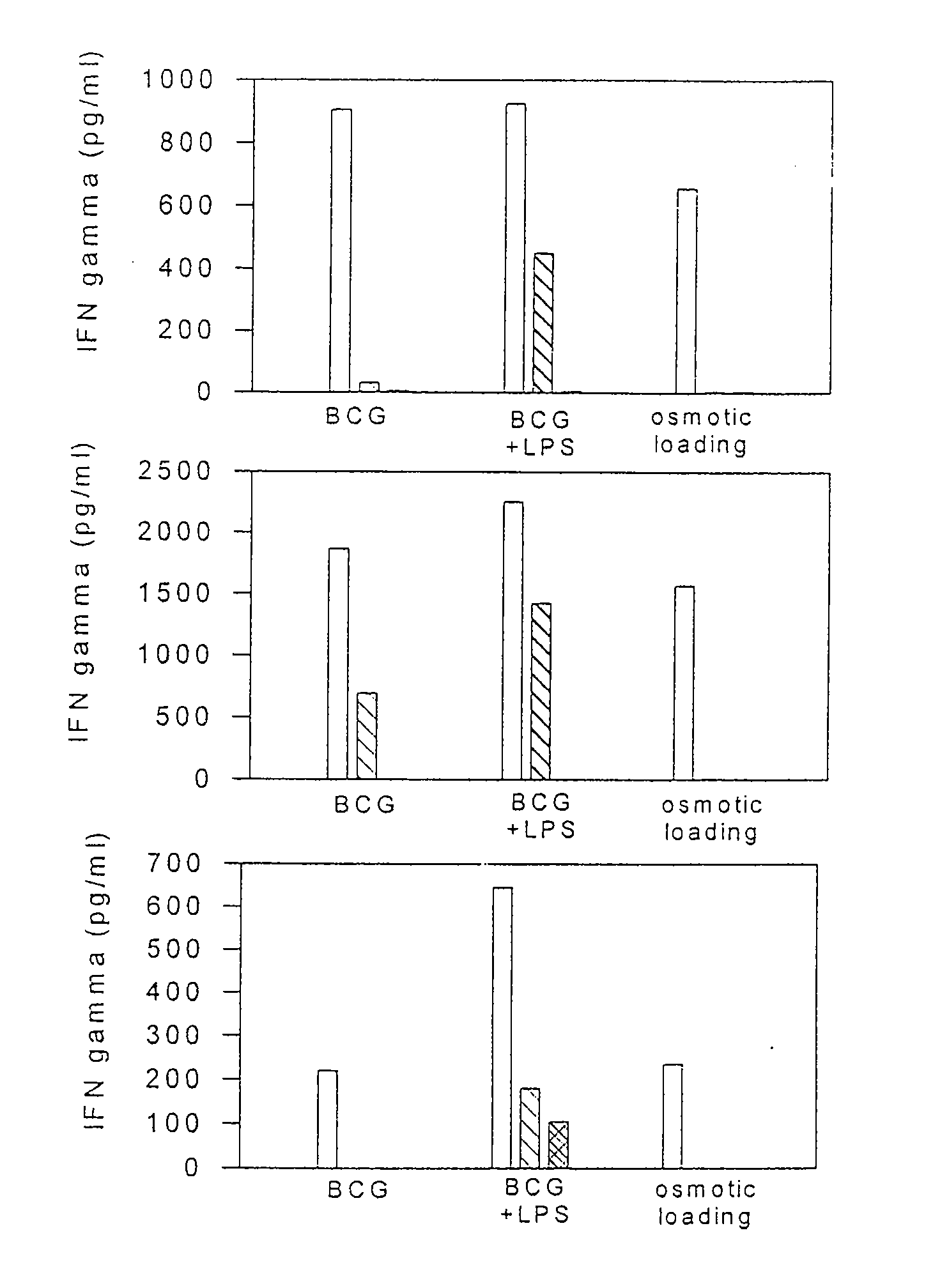
Method to increase class i presentation of exogenous antigens by human dendritic cells
InactiveUS20080171023A1Enhance MHC-class I processingEnhance immune responseBiocideArtificial cell constructsMHC class IDendritic cell
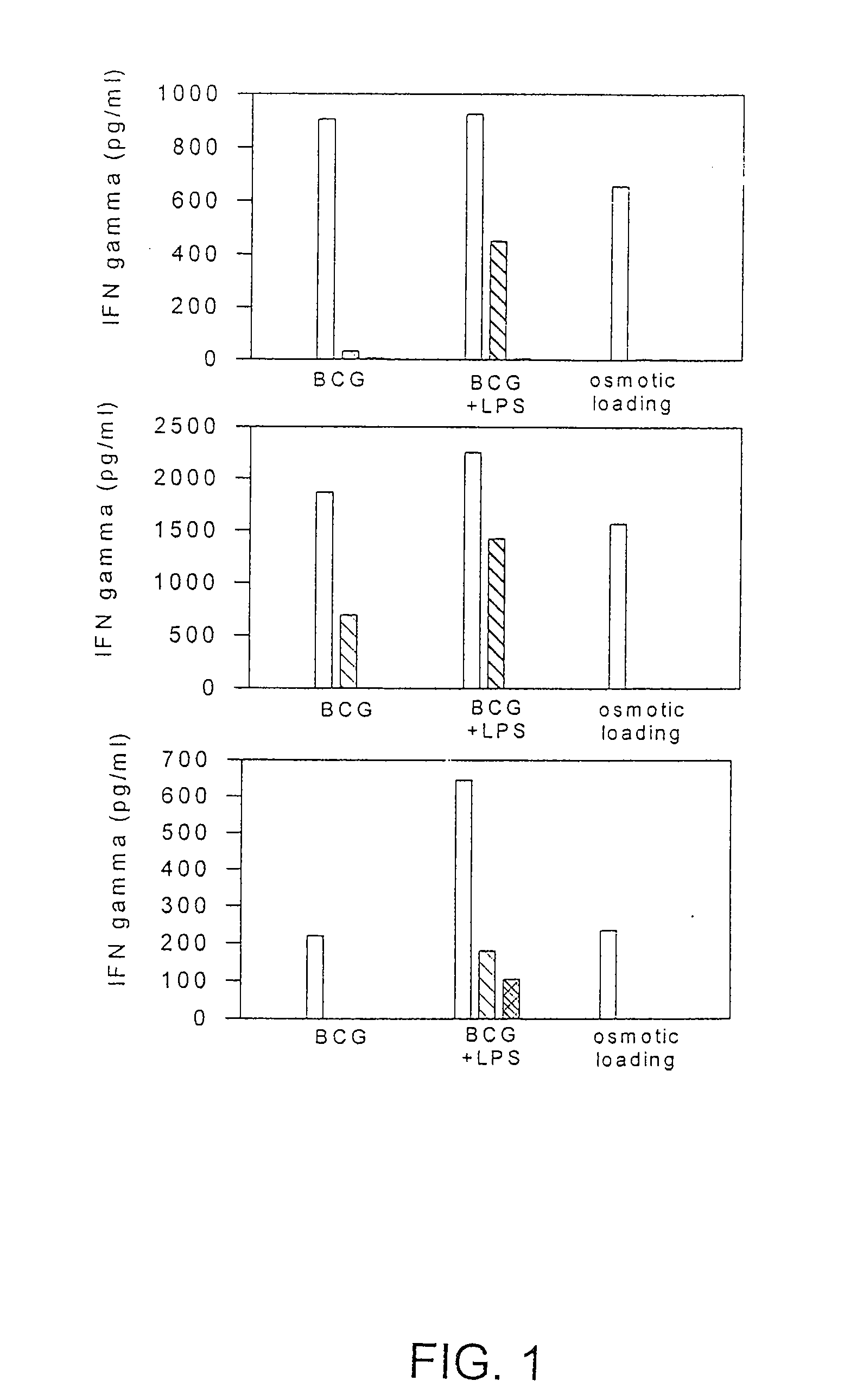
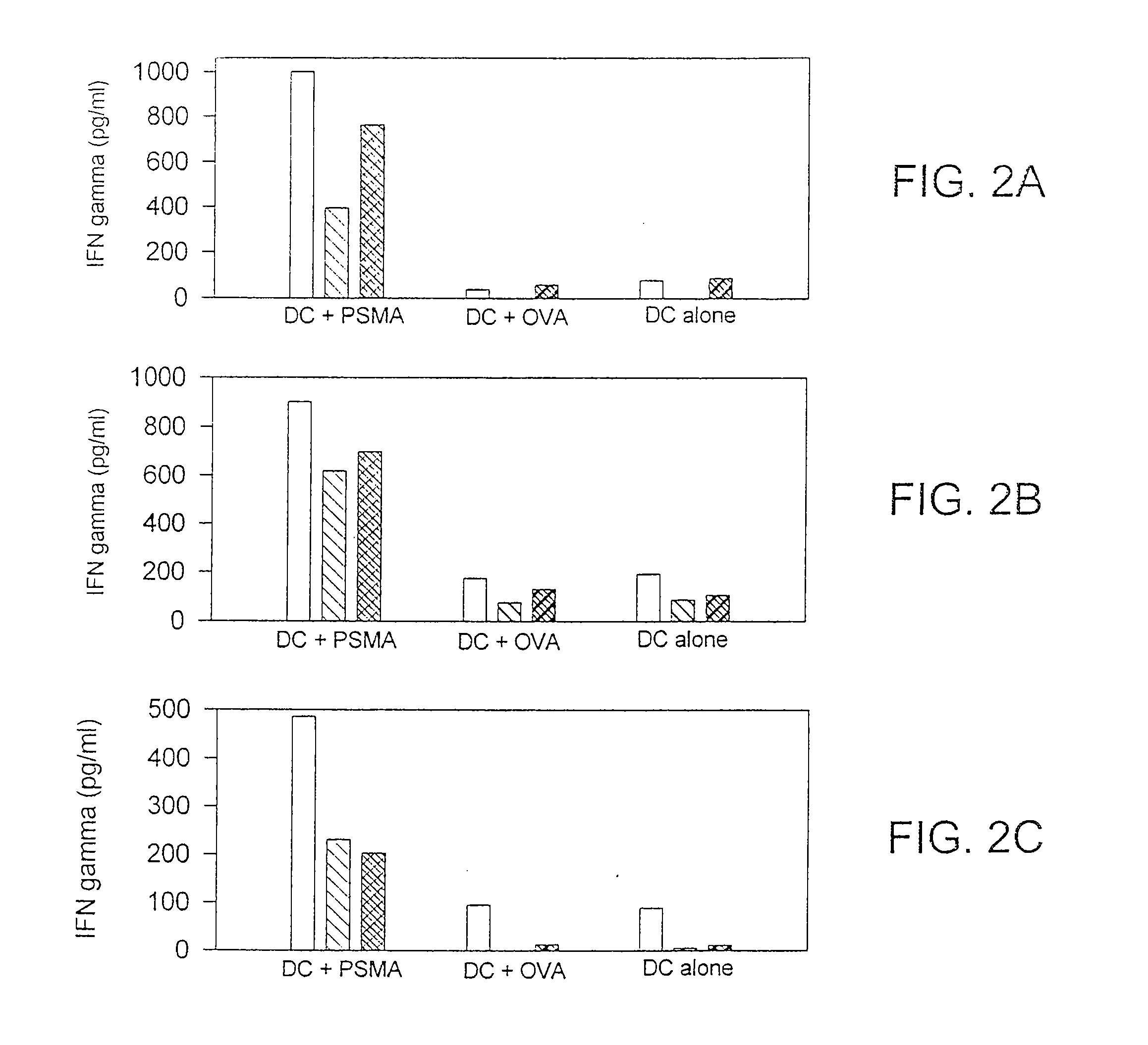
Methods and compositions for use of human dendritic cells to activate T cells for immunotherapeutic responses against primary and metastatic cancer are disclosed. In one embodiment, human dendritic cells exposed to a tumor associated antigen, or an antigenic fragment thereof in combination with bacillus Calmette-Guerin (BCG), are administered to a cancer patient to activate a predominantly CD8+T cell response in vivo. In an alternate embodiment, human dendritic cells are exposed to a tumor associated antigen or a specific antigenic peptide in combination with BCG in vitro and incubated or cultured with primed or unprimed T cells to activate a predominantly CD8+T cell response in vitro. The activated T cells are then administered to a cancer patient. Antigen in combination with BCG is processed by dendritic cells through the MHC-CLASS I compartment which provides for a predominantly CD8+T cell response. The addition of LPS provides for a greater number of mature dendritic cells enhancing the T cell response to antigen. Methods and compositions for human dendritic cells with extended life span and cryopreserved dendritic cells are disclosed.
Owner:NORTHWEST BIOTHERAPEUTICS INC
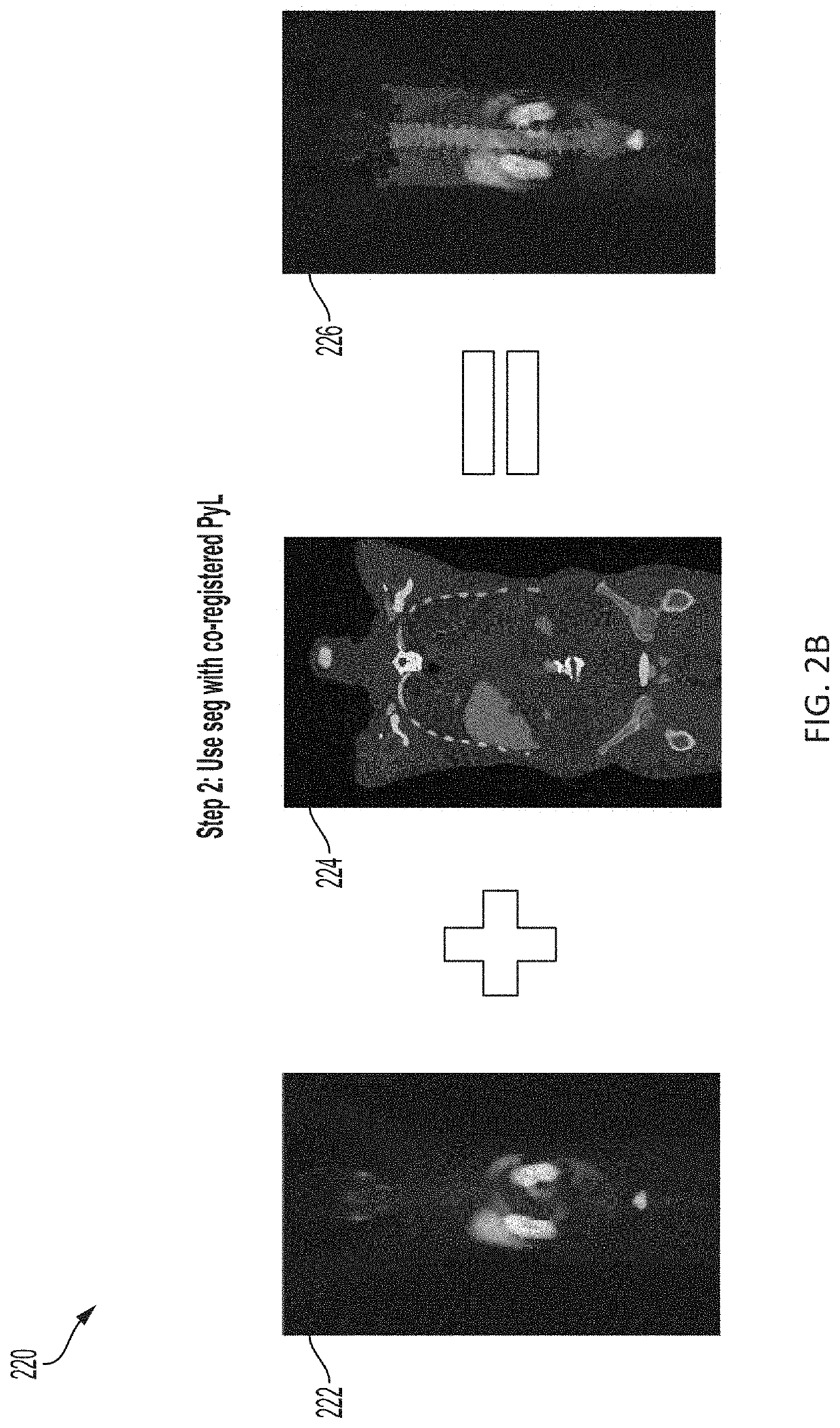
Systems and methods for artificial intelligence-based image analysis for cancer assessment
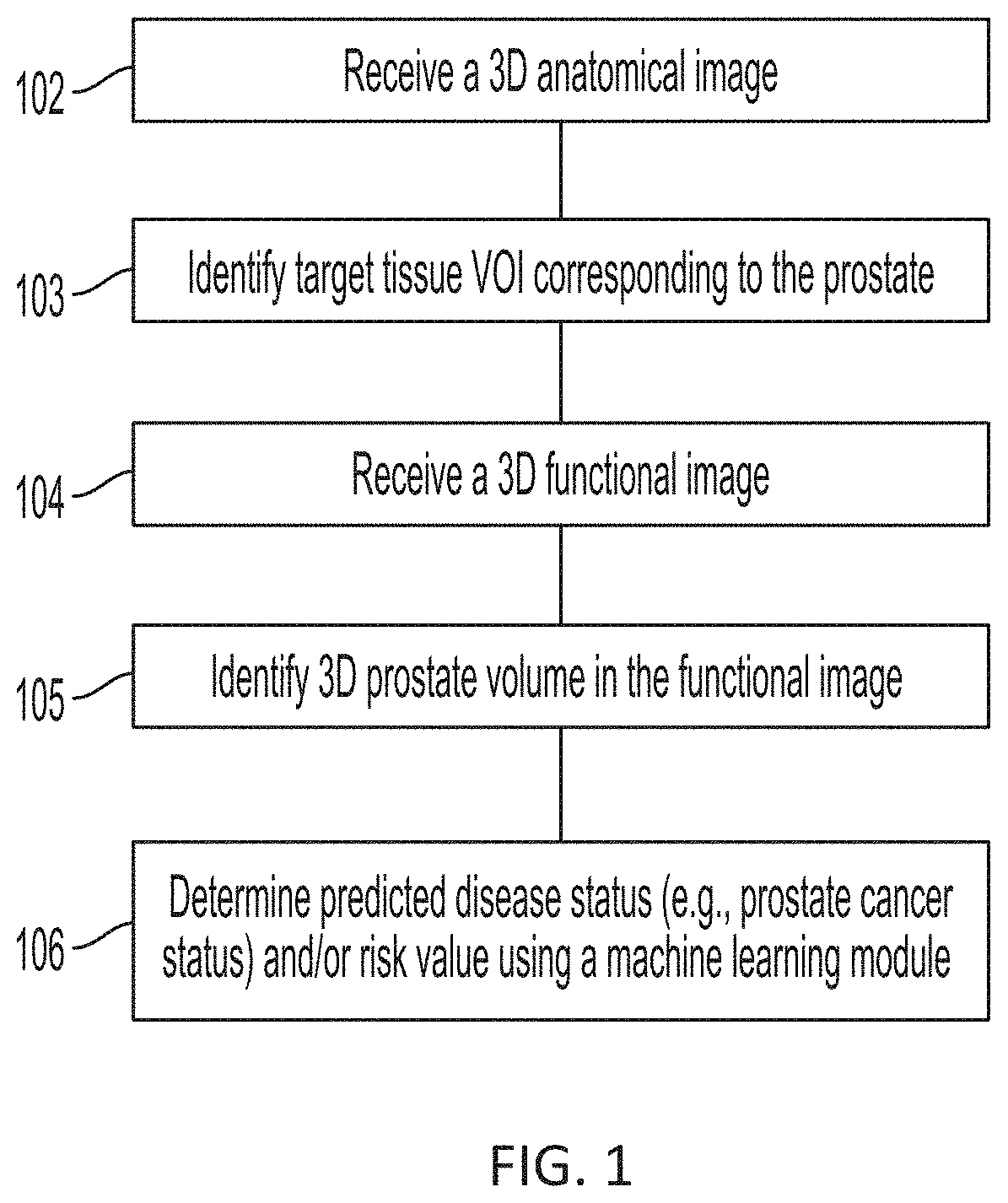
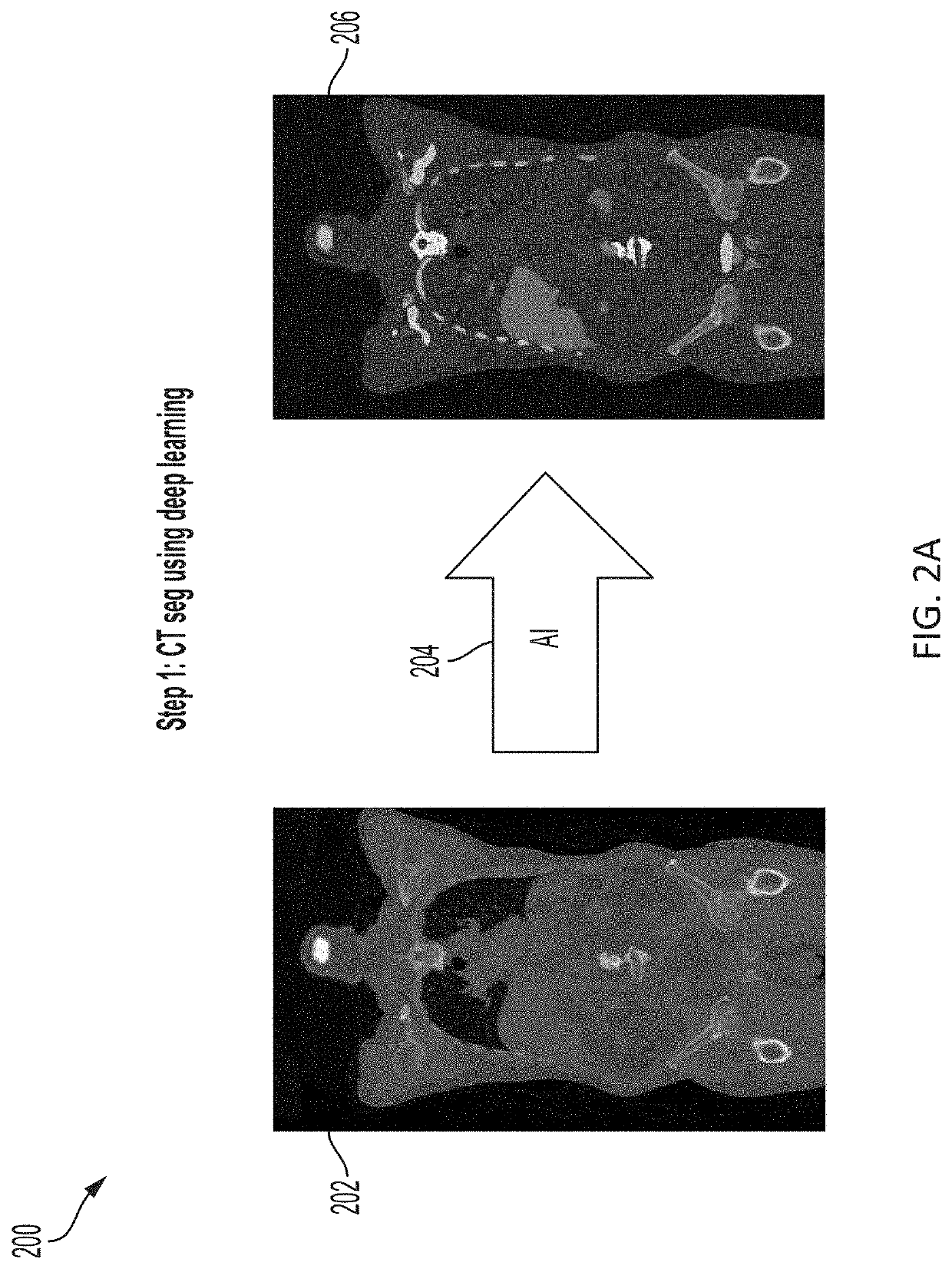
Presented herein are systems and methods that provide for automated analysis of medical images to determine a predicted disease status (e.g., prostate cancer status) and / or a value corresponding to predicted risk of the disease status for a subject. The approaches described herein leverage artificial intelligence (AI) to analyze intensities of voxels in a functional image, such as a PET image, and determine a risk and / or likelihood that a subject's disease, e.g., cancer, is aggressive. The approaches described herein can provide predictions of whether a subject that presents a localized disease has and / or will develop aggressive disease, such as metastatic cancer. These predictions are generated in a fully automated fashion and can be used alone, or in combination with other cancer diagnostic metrics (e.g., to corroborate predictions and assessments or highlight potential errors). As such, they represent a valuable tool in support of improved cancer diagnosis and treatment.
Owner:PROGENICS PHARMA INC +1
Diagnosis and treatment of cancer related to human dormancy
InactiveUS8075902B2Increase the gapEnhance cutaneous eliminationBioreactor/fermenter combinationsOrganic active ingredientsCancer cellMicrobial agent
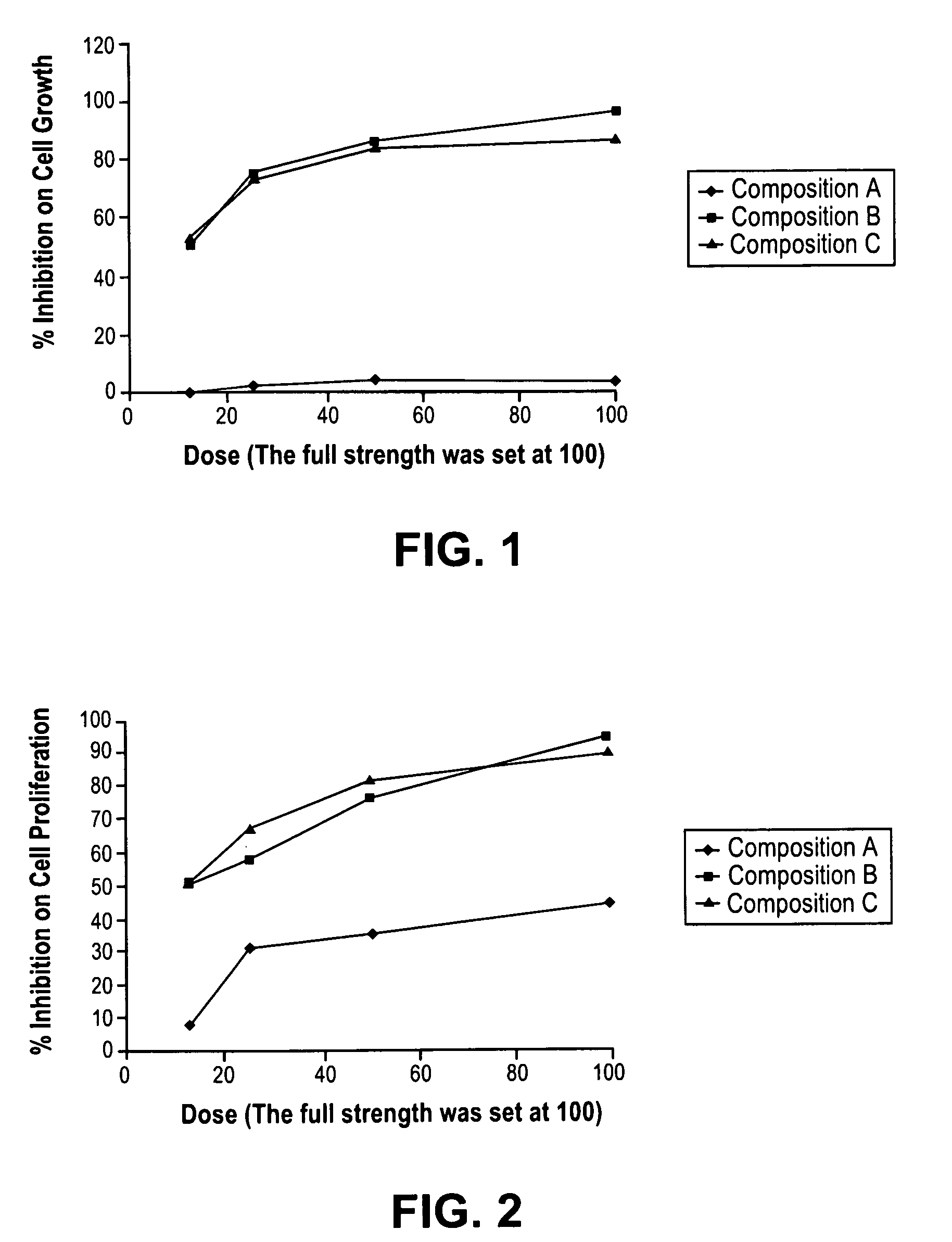
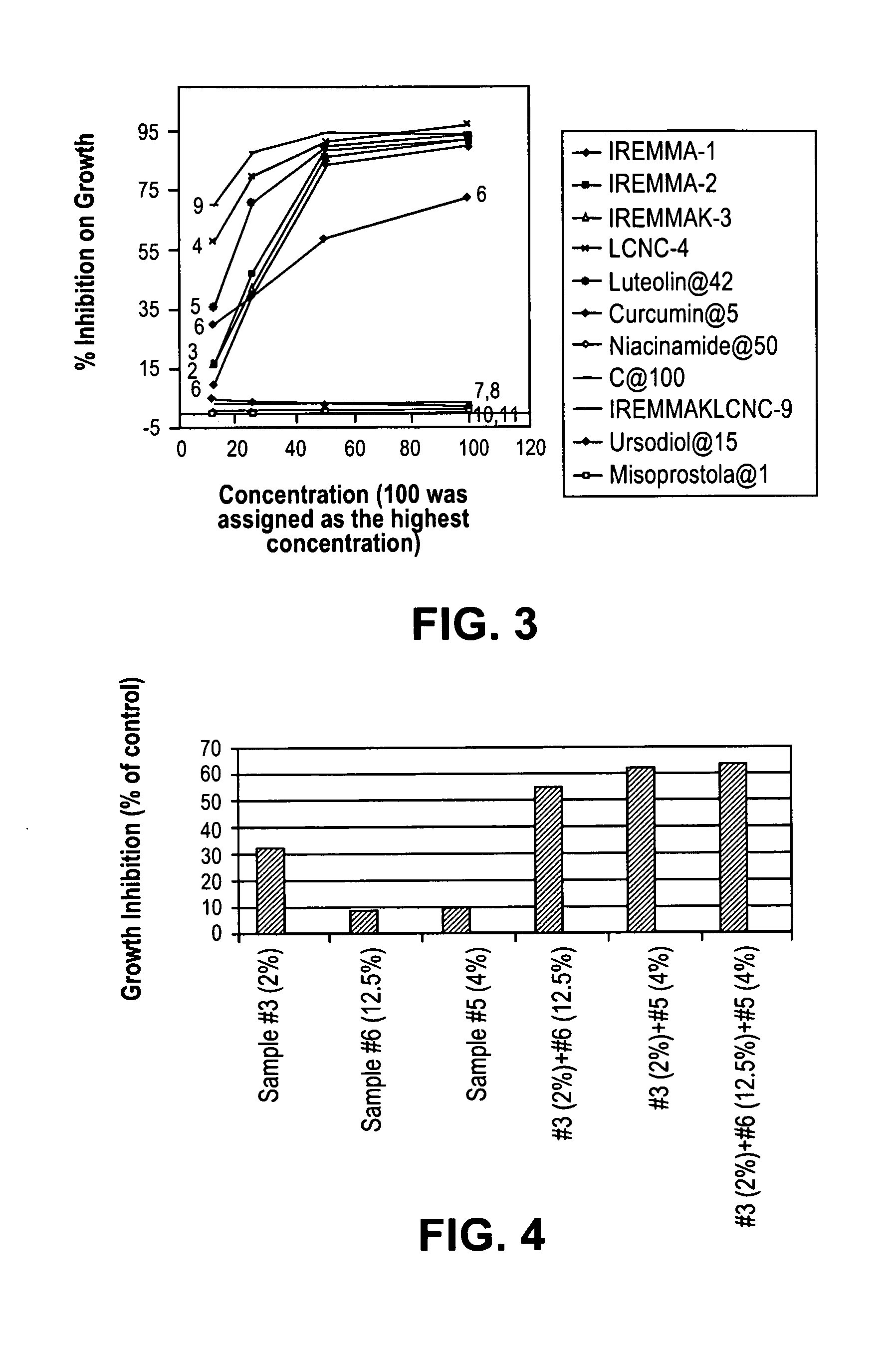
New devices and methods for diagnosis and compositions and methods for treatment of cancers use combinations of antimicrobial agents and agents that can reverse dormancy and hibernation pathways. We unexpectedly found that surprisingly low doses of anti-hibernation compounds can substantially inhibit cancer cell growth in vitro and can successfully treat cancers, including metastatic cancer. We also unexpectedly found that antimicrobial agents and anti-HDS compounds together can increase the degree of inhibition of cancer cell growth in a synergistic fashion. We conclude that combination therapy with antimicrobial agents and anti-HDS compounds can be effective in treating human patients with cancer.
Owner:POWELL CO LTD
Monoclonal antibodies for treatment of cancer
ActiveUS20130071325A1Minimize adverse effectsStrong formationAnimal cellsIn-vivo radioactive preparationsDiseaseMelanoma
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast cancer, lung cancer, gastric cancer, ovarian cancer, hepatocellular cancer, colon cancer, pancreatic cancer, esophageal cancer, head & neck cancer, kidney cancer, in particular renal cell carcinoma, prostate cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid cancer, and the metastatic forms thereof. In one embodiment, the tumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITATSMEDIZIN DER JOHANNES GUTENBERG UNIVERS +1
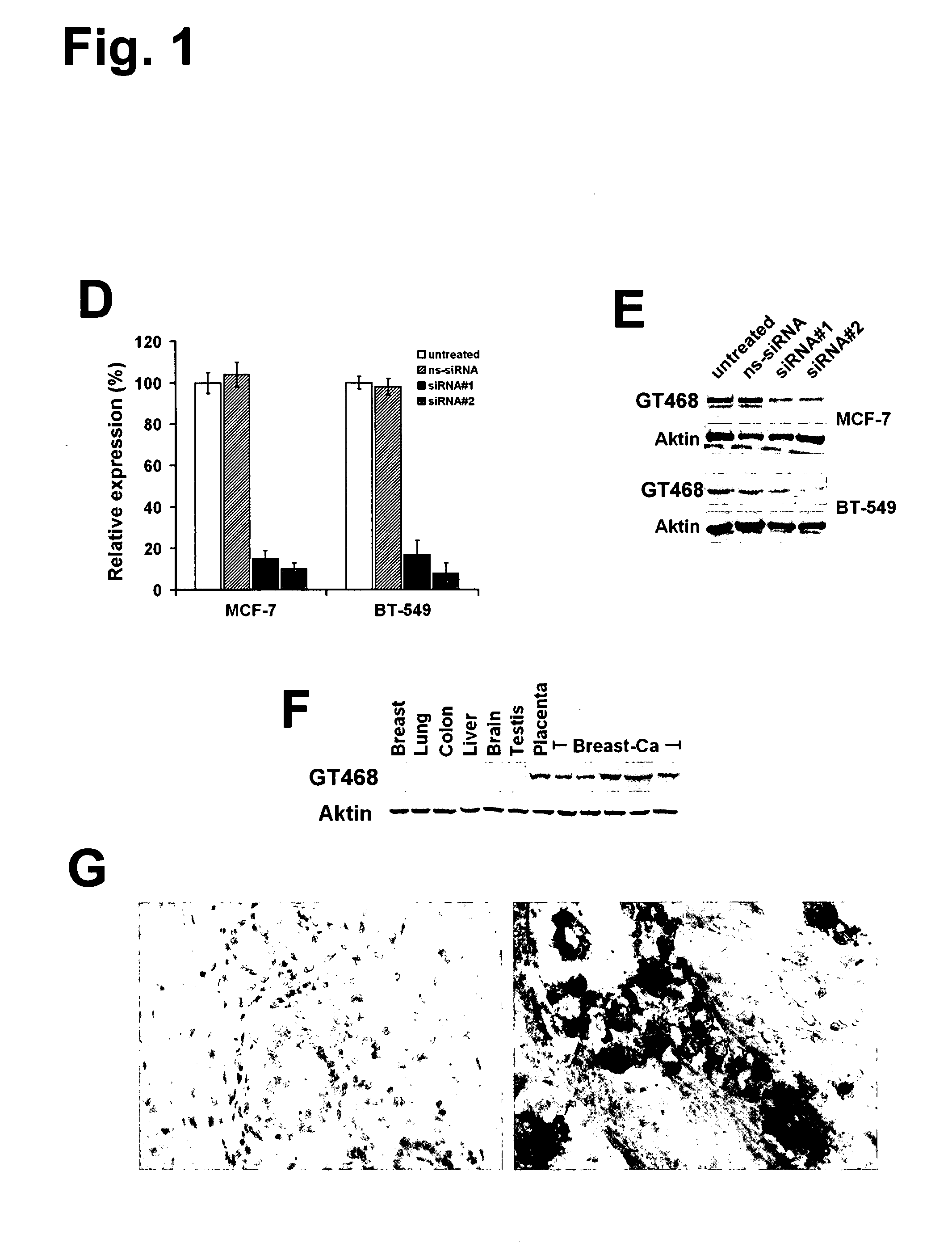
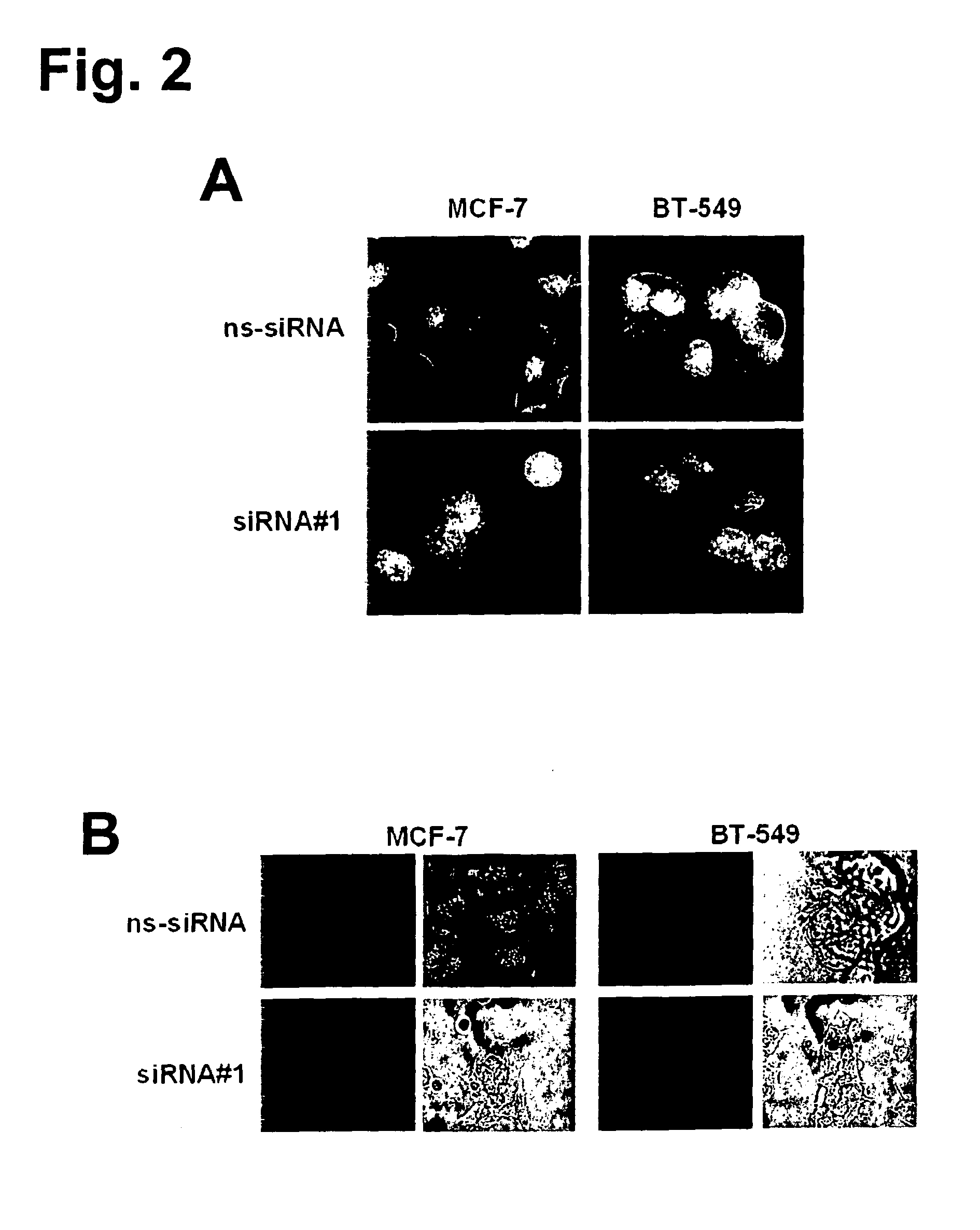
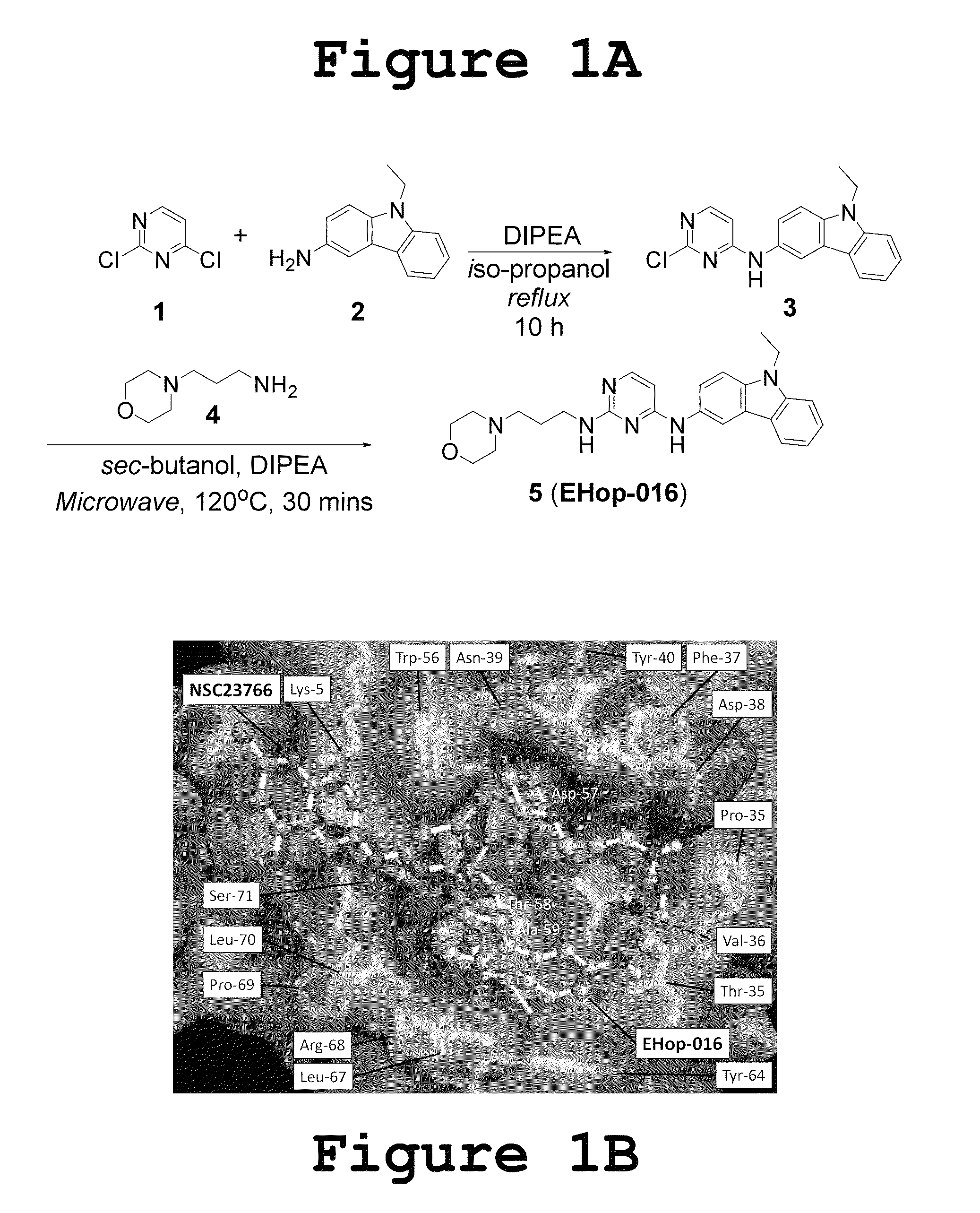
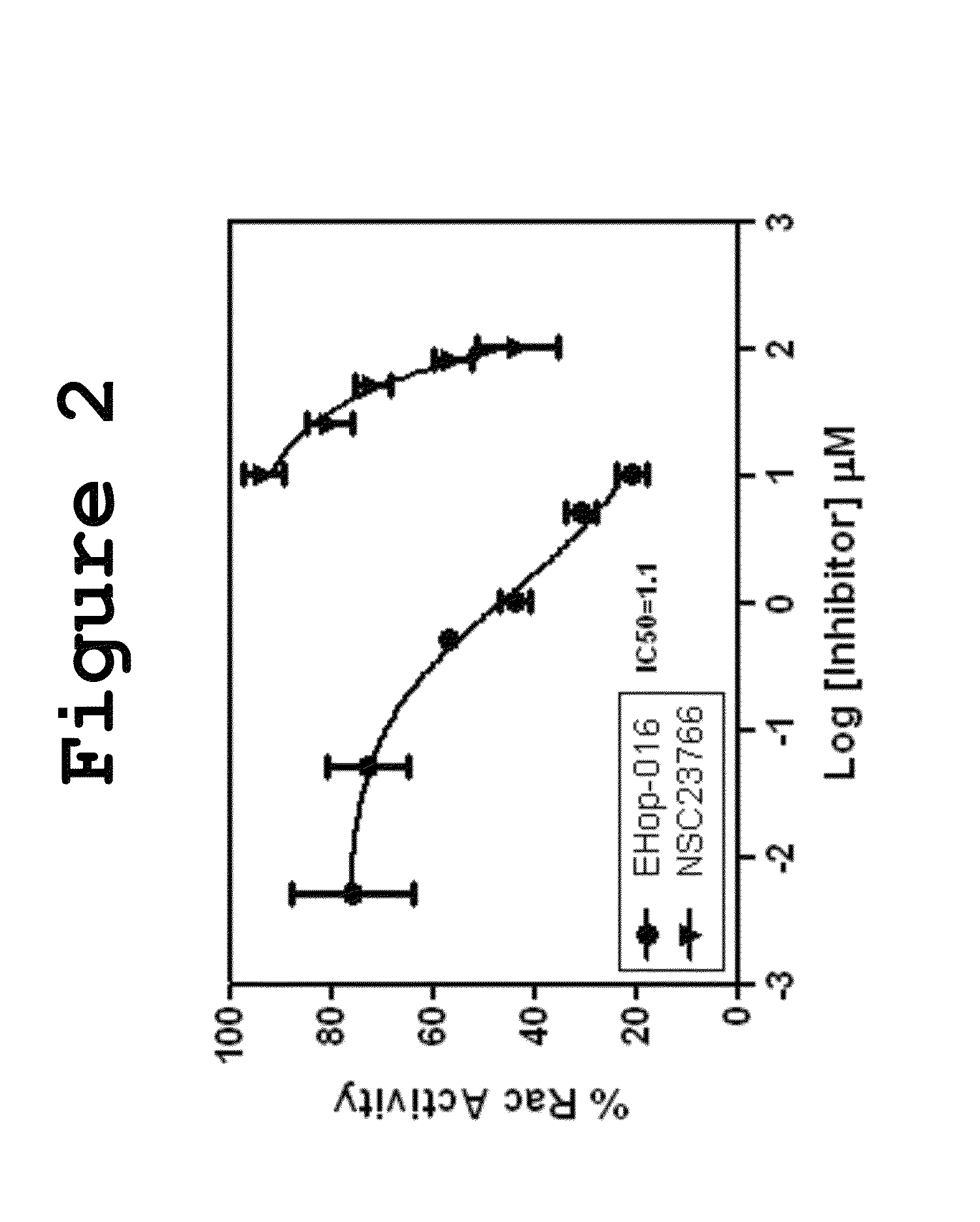
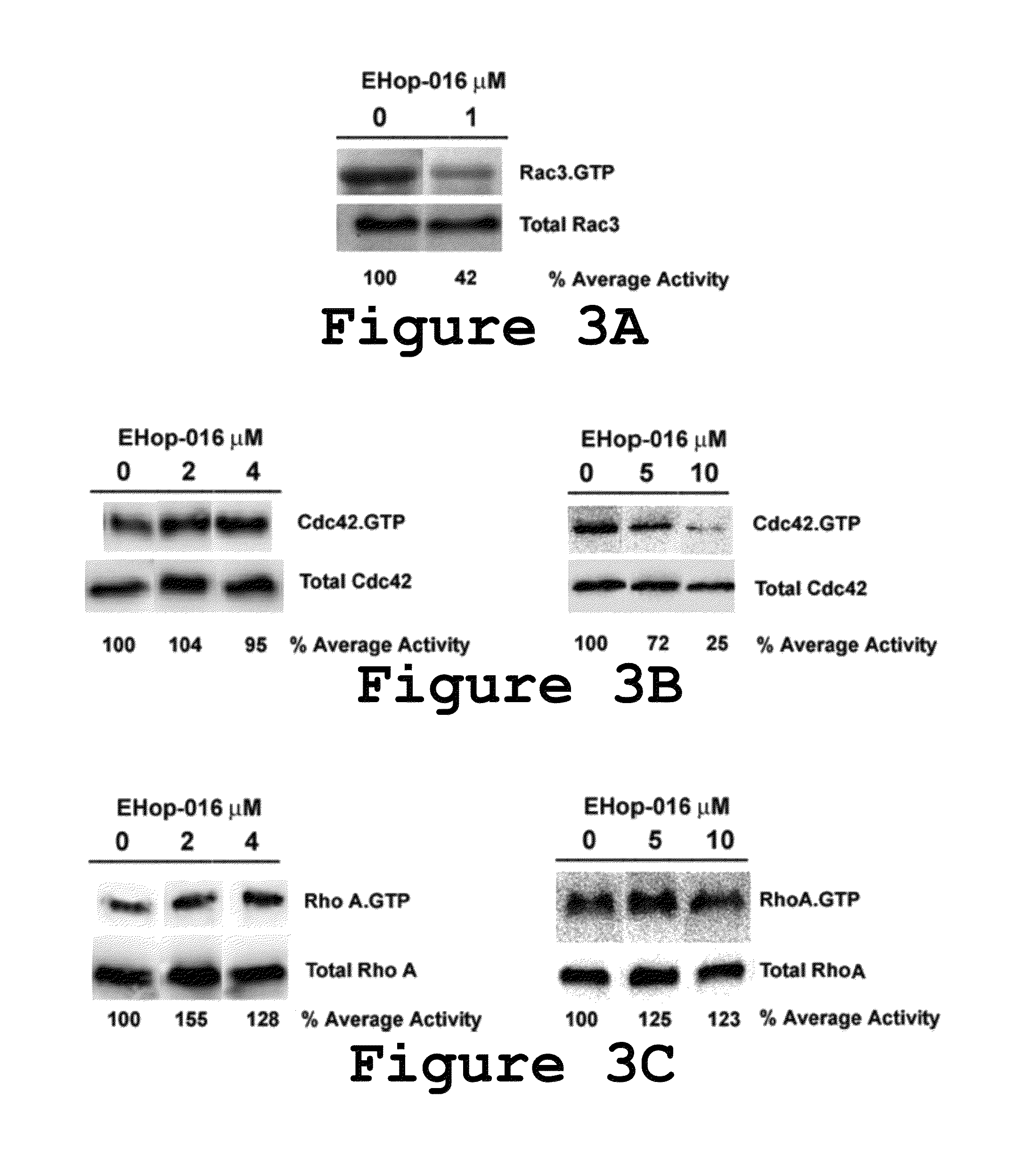
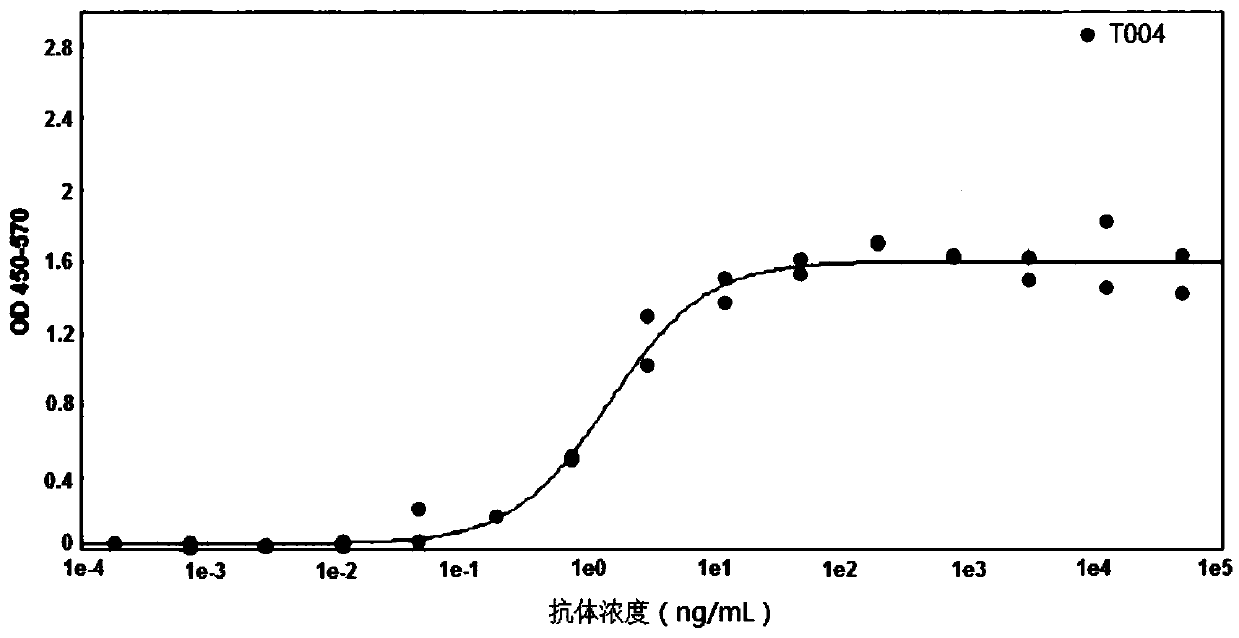
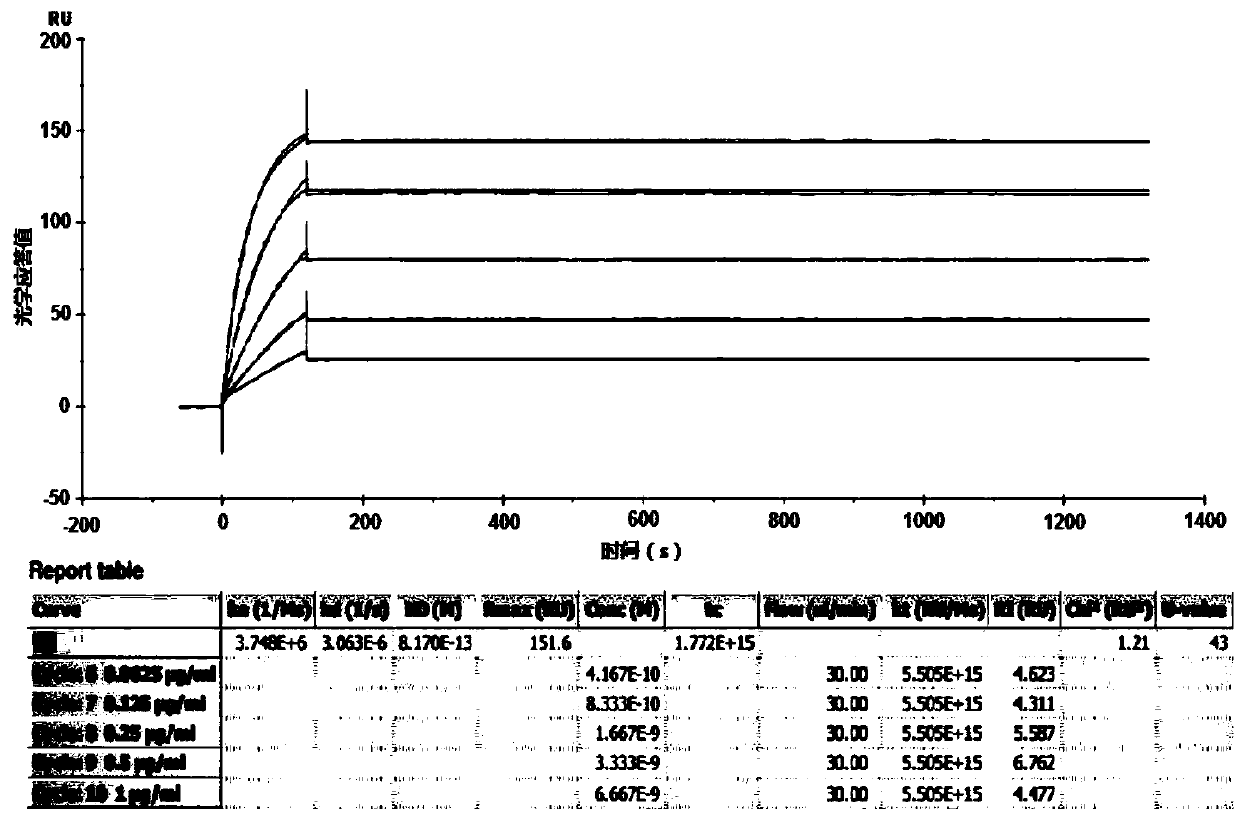
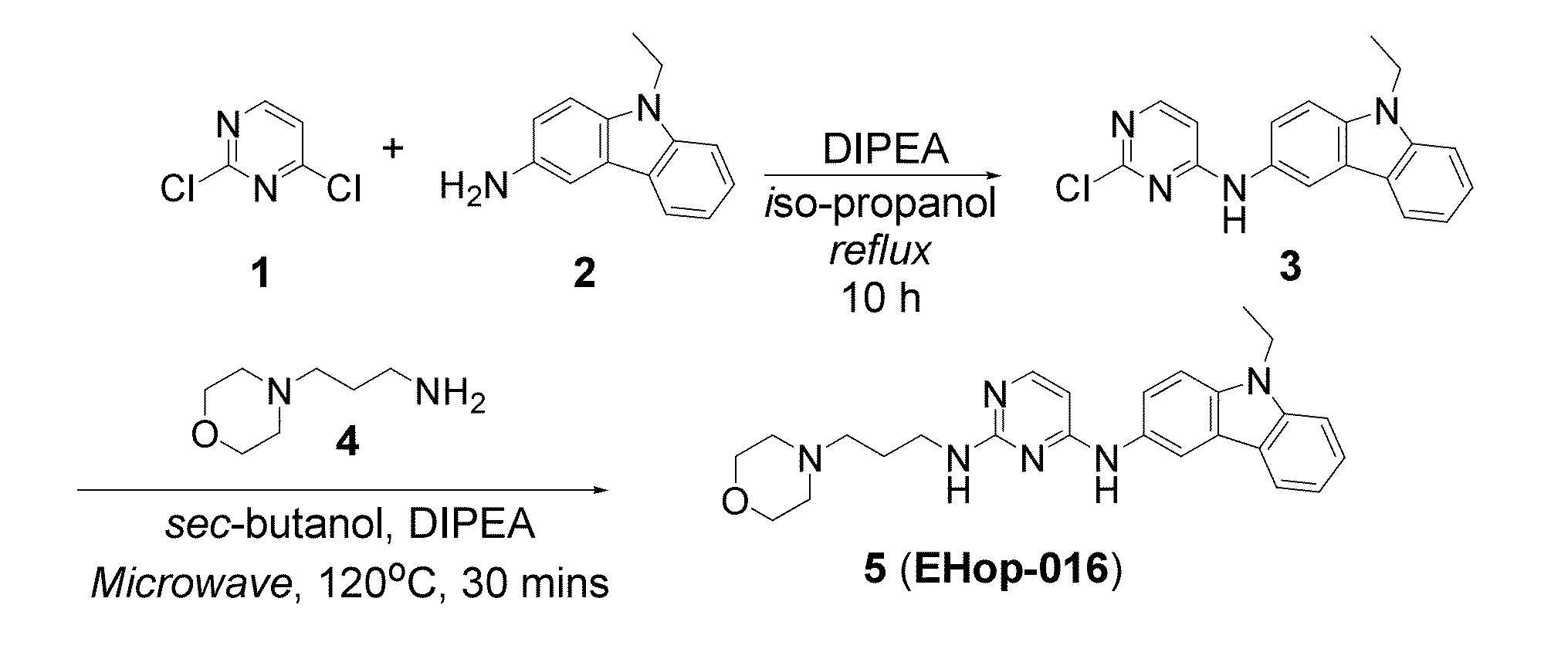
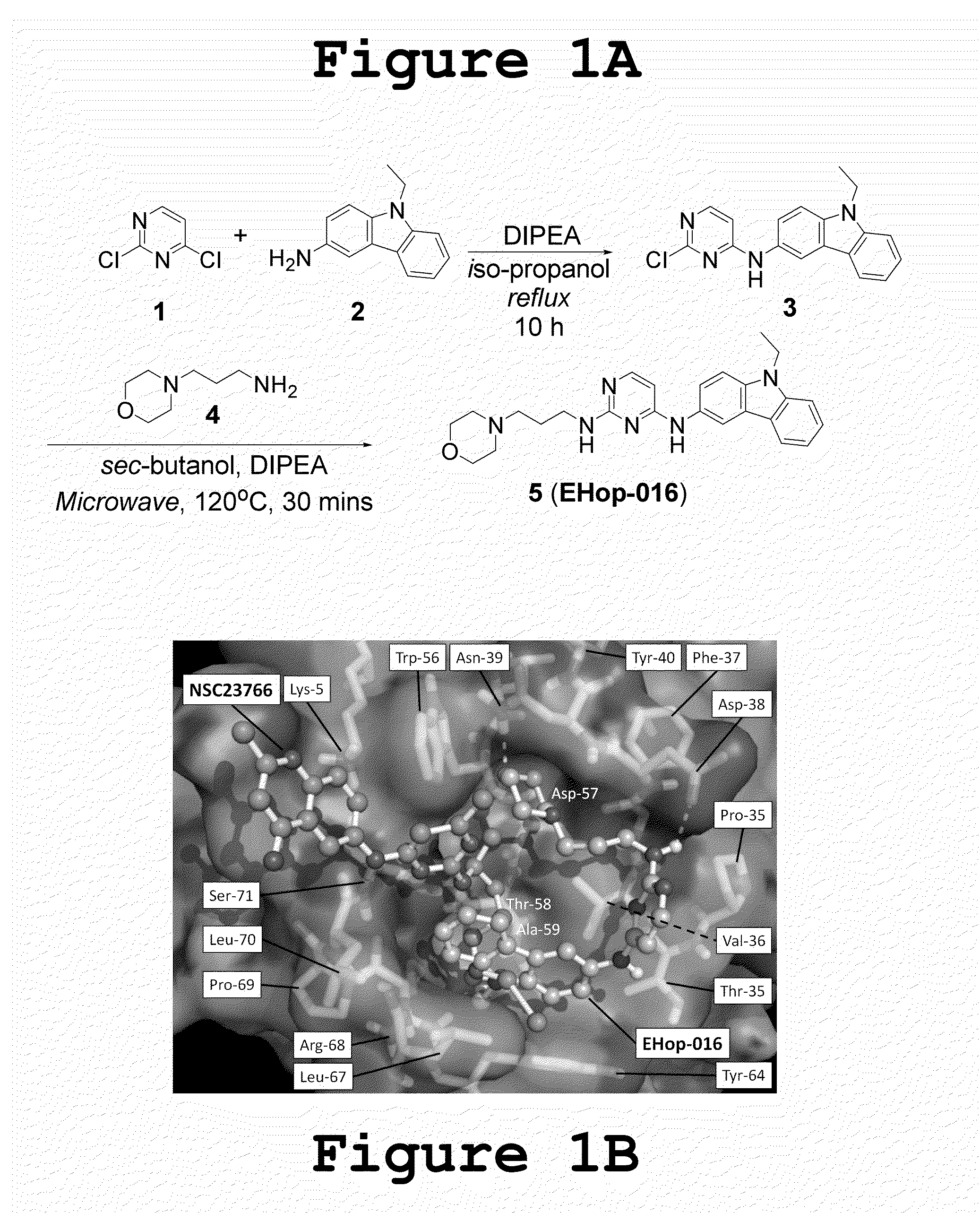
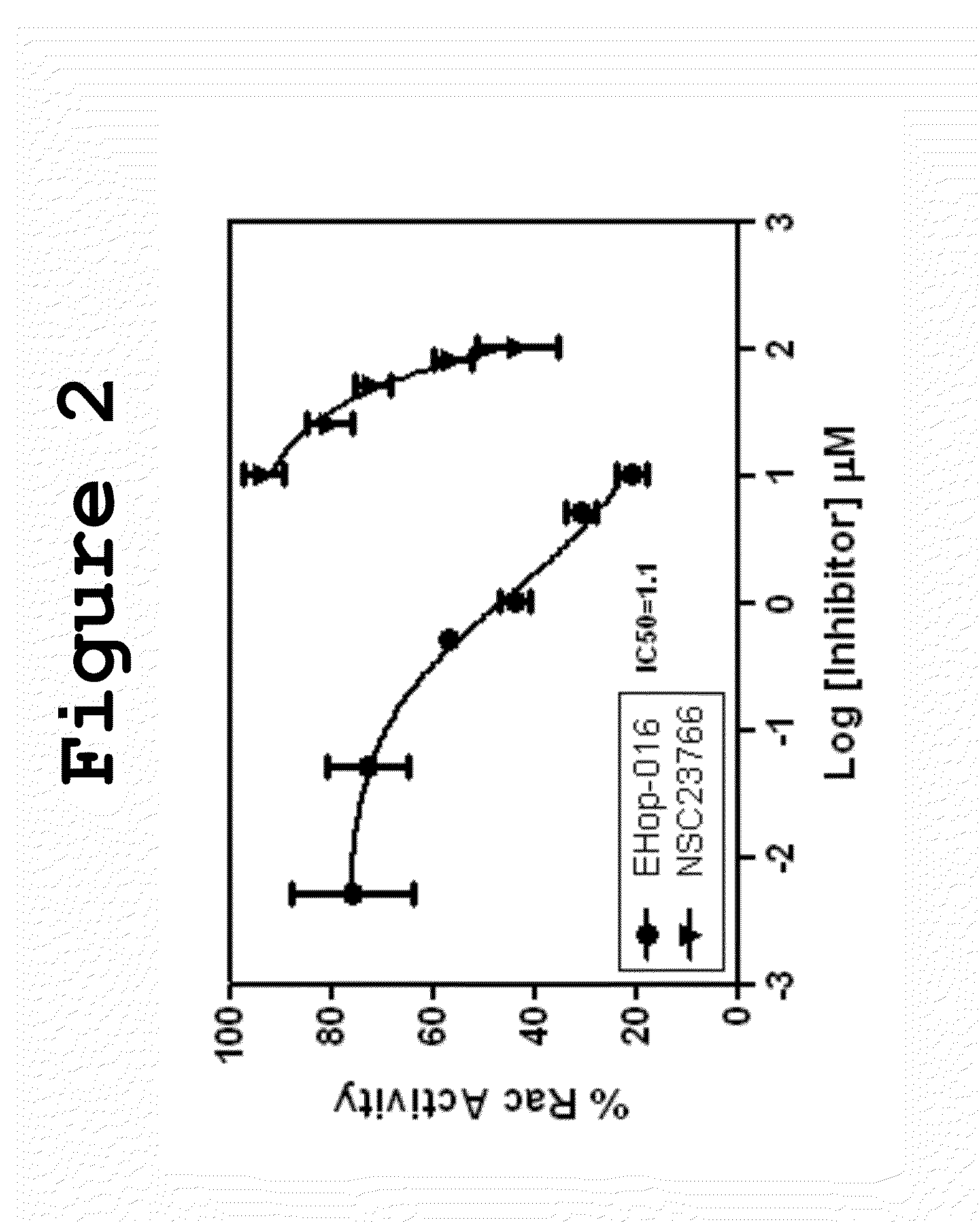
Small-molecule inhibitors of Rac1 in metastatic breast cancer
A novel inhibitor of Rac activity based on the structure of the established Rac / Rac-GEF inhibitor NSC23766 is discloses. The compound EHop-016, with an IC50 of 1.1 μM, is a 100-fold more efficient inhibitor of Rac activity than NSC23766. EHop-016 is specific for Rac1 and Rac3 at concentrations ≦5 mM. At higher concentrations, EHop-016 inhibits the close homolog Cdc42. In MDA-MB-435 cells, EHop-016 (≦5 mM) inhibits the association of the Rac-GEF Vav2 with a nucleotide-free Rac1(G15A), which has a high affinity for activated GEFs. EHop-016 does not affect the association of the Rac-GEF Tiam-1 with Rac1(G15A) at similar concentrations. EHop-016 also inhibits the Rac activity of MDA-MB-231 metastatic breast cancer cells and reduces Rac-directed lamellipodia formation in both cell lines. EHop-016 decreases Rac-downstream effects of p21-activated kinase (PAK)1 activity and directed migration of metastatic cancer cells. At low concentrations (<5 μM), EHop-016 does not affect cell viability.
Owner:UNIVERSITY OF PUERTO RICO
Methods and compositions for treating metastatic breast cancer and other cancers in the brain
ActiveUS20170043035A1Decreased and no measurable affinityShrink tumorNervous disorderPharmaceutical delivery mechanismRegimenWilms' tumor
A composition comprising at least one AAV vector formulated for central nervous system delivery is described. The composition comprises at least one expression cassette which contains sequences encoding an anti-neoplastic immunoglobulin construct for delivery to the brain operably linked to expression control sequences therefor and a pharmaceutically acceptable carrier. The anti-neoplastic immunoglobulin construct may be an immunoglobulin modified to have decreased or no measurable affinity for neonatal Fc receptor (FcRn). Also provided are methods of using these constructs in preparing pharmaceutical compositions and uses thereof in anti-neoplastic regimens, particularly for primary and / or metastatic cancers of the brain.
Owner:THE TRUSTEES OF THE UNIV OF PENNSYLVANIA
EpA2 monoclonal antibodies and methods of use thereof
InactiveUS20070166314A1Lower Level RequirementsIncreased phosphorylationOrganic active ingredientsFungiNon cancerCancer prevention
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and agonizes EphA2, thereby increasing EphA2 phosphorylation and decreasing EphA2 levels. In other embodiments, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and inhibits cancer cell colony formation in soft agar, inhibits tubular network formation in three-dimensional basement membrane or extracellular matrix preparation, preferentially binds to an EphA2 epitope that is exposed on cancer cells but not non-cancer cells, and / or has a low Koff, thereby, inhibiting tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA2 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
VEGFR2-targeting metastatic cancer vaccine
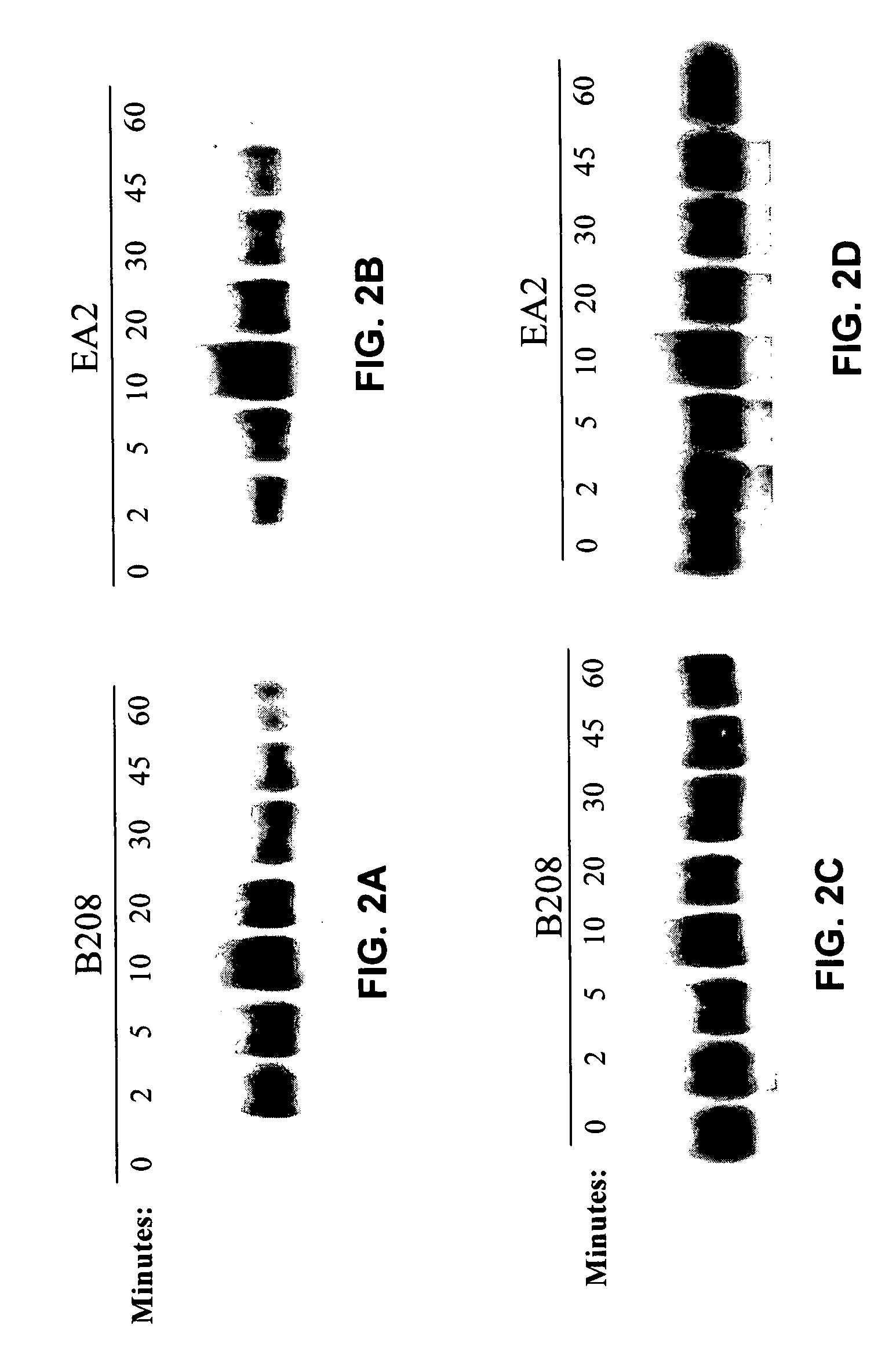
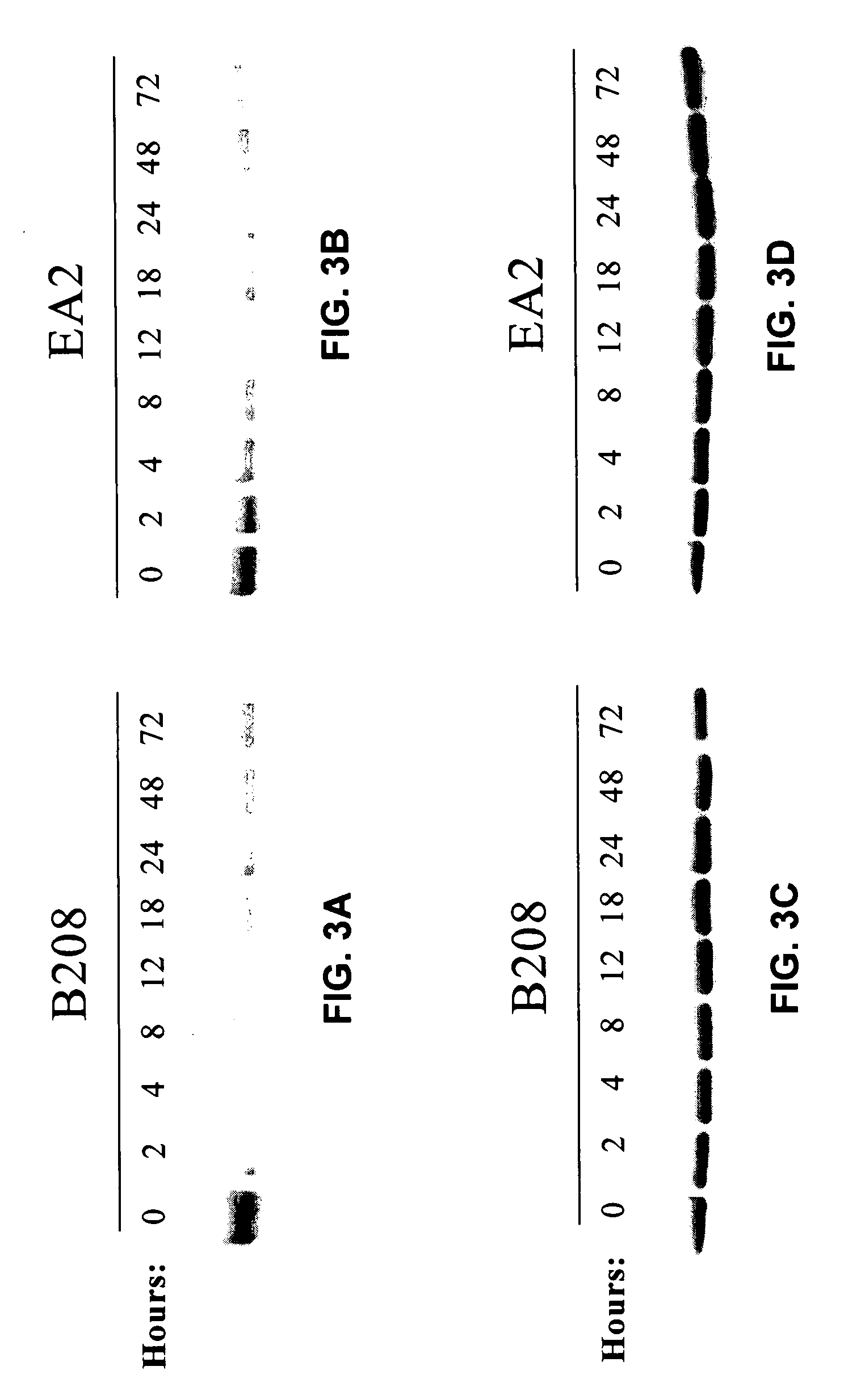
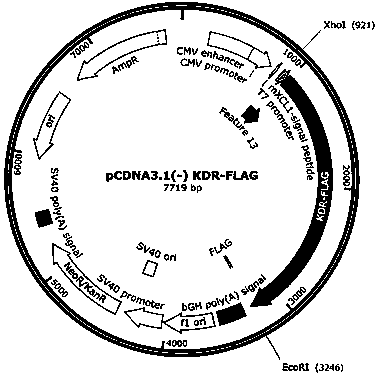
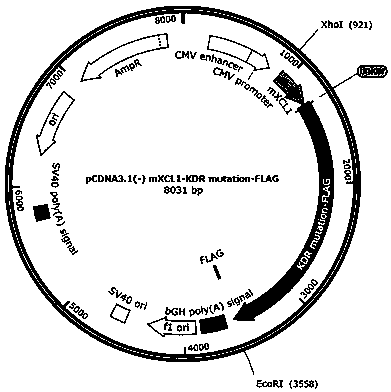
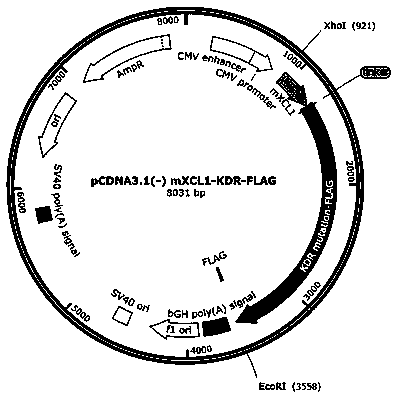
ActiveCN111440244AGrowth inhibitionEffective combinationChemokinesAntibody mimetics/scaffoldsTumor angiogenesisOncology
The invention relates to the fields of immunotherapy and prevention of cancer, in particular to a VEGFR2 (KDR)-targeting metastatic cancer vaccine. Through fusion expression of VEGFR2 protein promoting generation of tumor angiogenesis with a DC cell ligand XCL1, the efficiency of cytophagy, processing and presentation of the VEGFR2 protein by DC cells is improved, and the effect of inhibiting tumor growth is improved. Experiments prove that the protein for nucleic acid vaccine expression can effectively bind the DC cells, induce specific T-cell reaction of VEGR2 and significantly inhibit growth of tumor highly expressing VEGFR2 in multiple models.
Owner:NEWISH TECH (BEIJING) CO LTD +1
Tissue chip for detecting backbone metastatic carcinoma prognosis related molecule sign
InactiveCN101013086AImprove clinical treatment effectRational treatment strategyMicrobiological testing/measurementColor/spectral properties measurementsCancer developmentLarge sample
The invention relates to the biotechnology area, and the spinal metastatic cancer prognosis judgment is the key to correct determine clinical development strategy, and in the invention, for the present spinal metastatic cancer related molecular biology prognosis judgment evaluation lacking problem, it constructs the spinal metastatic cancer prognosis related molecular marker tissue chip. By screening the clinical data and determining the random data spinal metastatic cancer pathological wax block to produce the spinal metastatic cancer tissue chip; selecting a variety of molecular markers and tissue chips for immunohistochemistry or in-situ hybridization, and through image analysis and quantitative measurement to analyze the expression instance of each molecular marker in the spinal metastatic cancer pathological specimens, and to analyze the relationship between the molecular markers expression and the patients prognosis, and screening the molecular markers which are closely related to the spinal metastatic cancer prognosis. The tissue chip provides a high-throughput, large sample, rapid detection tool for screening the spinal metastatic cancer prognostic related molecular markers, and provides a theoretical basis for the rational determination of the spinal metastatic cancer development strategies.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Monoclonal antibodies for treatment of cancer
ActiveUS9216218B2Strong formationStrong proliferationAnimal cellsHybrid immunoglobulinsDiseaseAntiendomysial antibodies
The present invention provides antibodies useful as therapeutics for treating and / or preventing diseases associated with cells expressing GT468, including tumor-related diseases such as breast cancer, lung cancer, gastric cancer, ovarian cancer, hepatocellular cancer, colon cancer, pancreatic cancer, esophageal cancer, head & neck cancer, kidney cancer, in particular renal cell carcinoma, prostate cancer, liver cancer, melanoma, sarcoma, myeloma, neuroblastoma, placental choriocarcinoma, cervical cancer, and thyroid cancer, and the metastatic forms thereof. In one embodiment, the tumor disease is metastatic cancer in the lung.
Owner:TRON TRANSLATIONALE ONKOLOGIE AN DER UNIVERSITAETSMEDIZIN DER JOHANNES GUTENBERG UNIV MAINZ GEMEINNUETZIGE GMBH +1
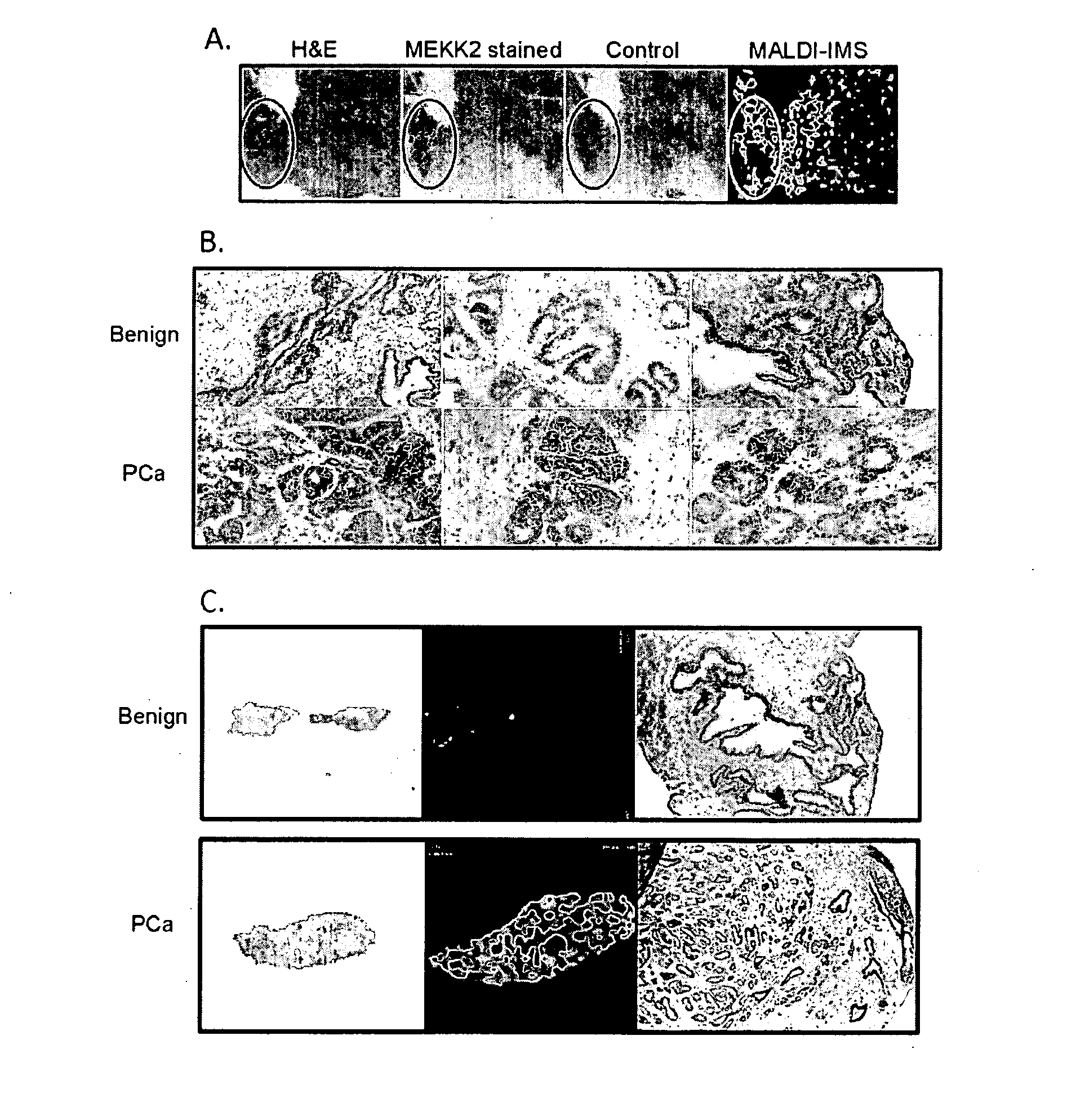
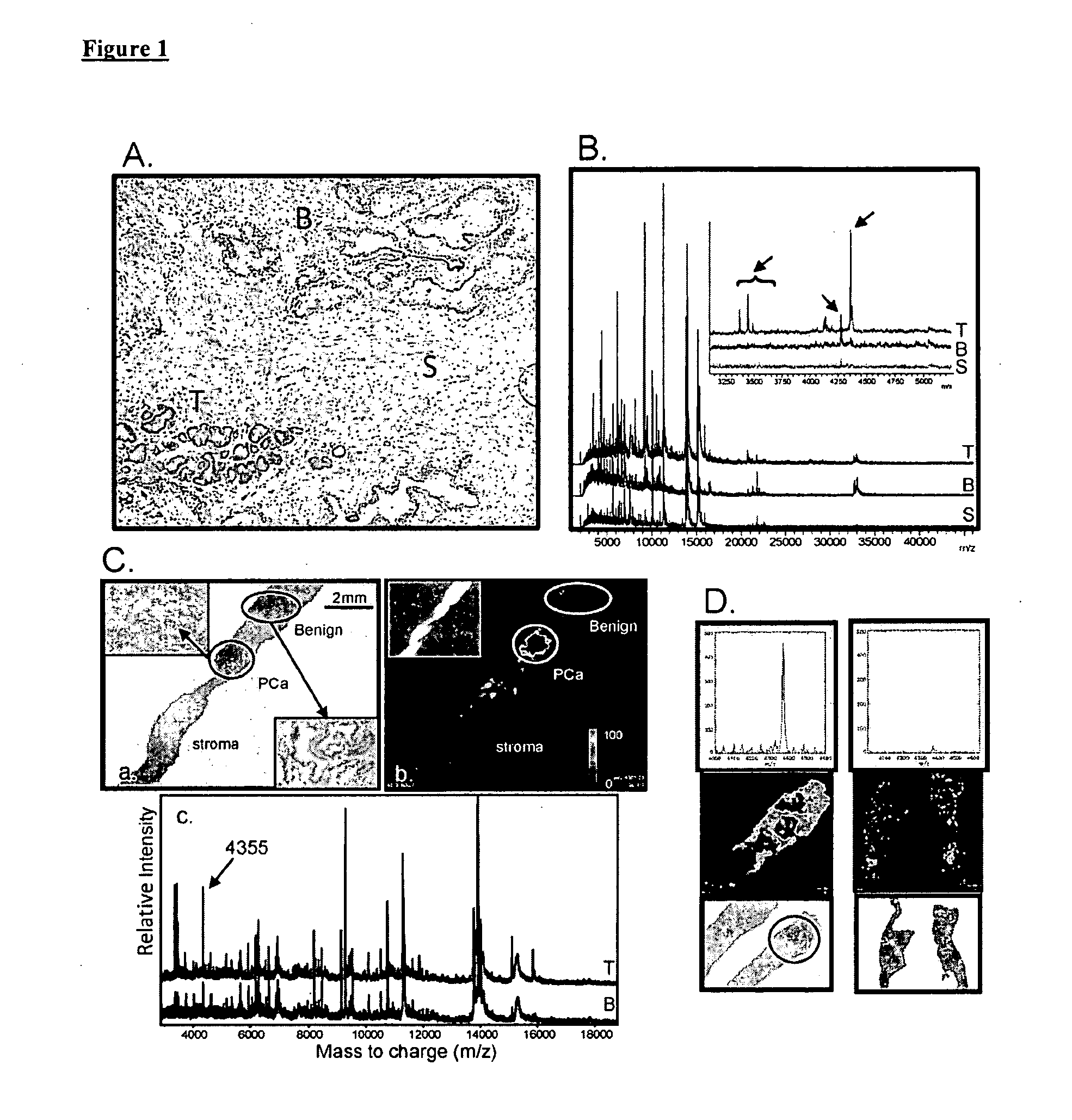
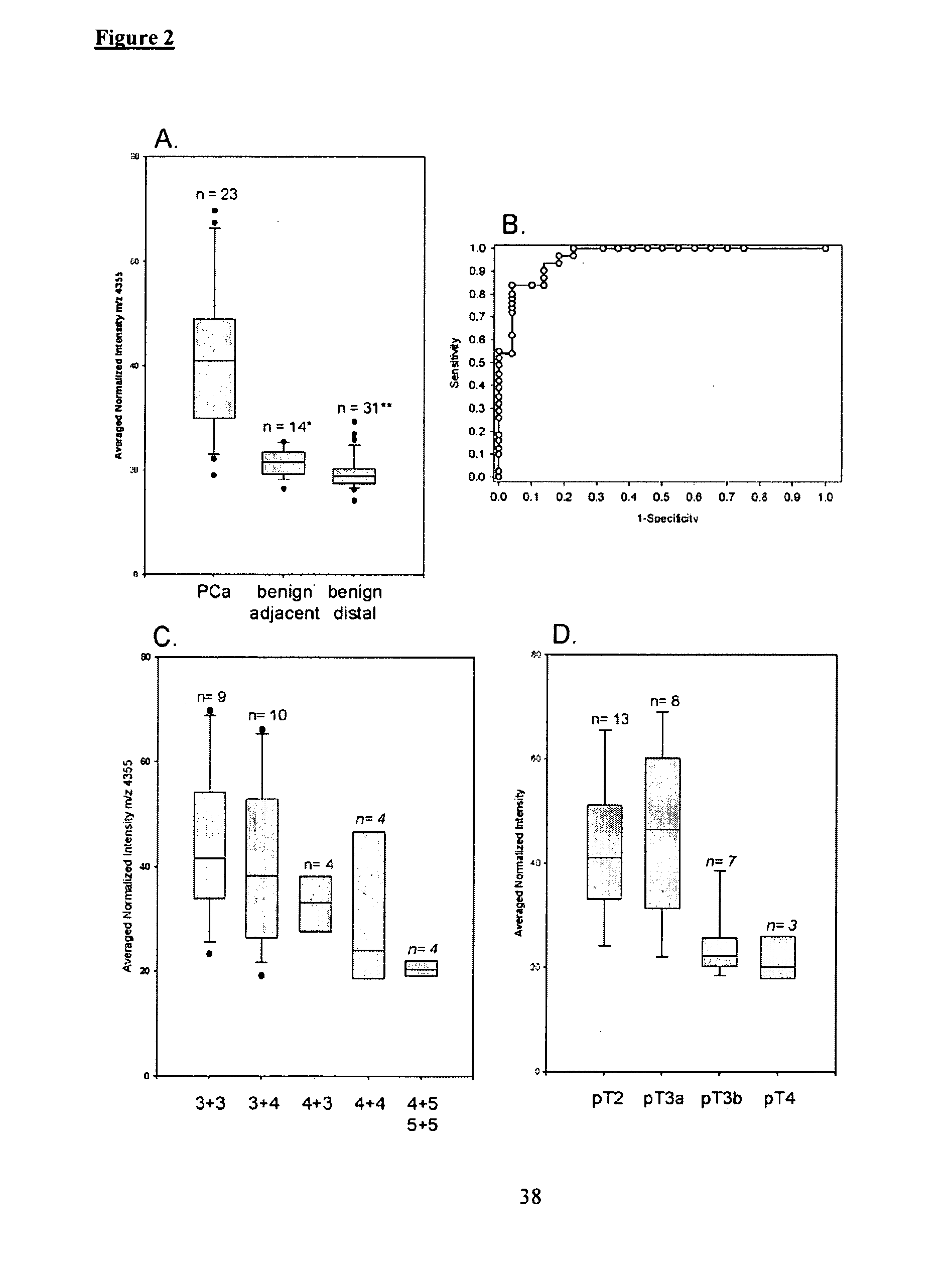
Imaging Mass Spectrometry for Improved Prostrate Cancer Diagnostics
InactiveUS20110136166A1Microbiological testing/measurementPreparing sample for investigationMass spectrometryDisease
The invention provides biomarkers that can discriminate between prostate cancer and normal tissue as well as identification of associated metastatic disease. One biomarker was identified as a peptide fragment of MEKK2. Methods of diagnosing prostate cancer, including metastatic cancer, by detecting the differential expression of one or more biomarkers are also provided.
Owner:EASTERN VIRGINIA MEDICAL SCHOOL
Epha2 monoclonal antibodies and methods of use thereof
InactiveUS20100278838A1Lower Level RequirementsReduce proliferationOrganic active ingredientsFungiCancer preventionAntiendomysial antibodies
The present invention relates to methods and compositions designed for the treatment, management, or prevention of cancer, particularly, metastatic cancer. In one embodiment, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and agonizes EphA2, thereby increasing EphA2 phosphorylation and decreasing EphA2 levels. In other embodiments, the methods of the invention comprise the administration of an effective amount of an antibody that binds to EphA2 and inhibits cancer cell colony formation in soft agar, inhibits tubular network formation in three-dimensional basement membrane or extracellular matrix preparation, preferentially binds to an EphA2 epitope that is exposed on cancer cells but not non-cancer cells, and / or has a low Koff, thereby, inhibiting tumor cell growth and / or metastasis. The invention also provides pharmaceutical compositions comprising one or more EphA2 antibodies of the invention either alone or in combination with one or more other agents useful for cancer therapy.
Owner:MEDIMMUNE LLC
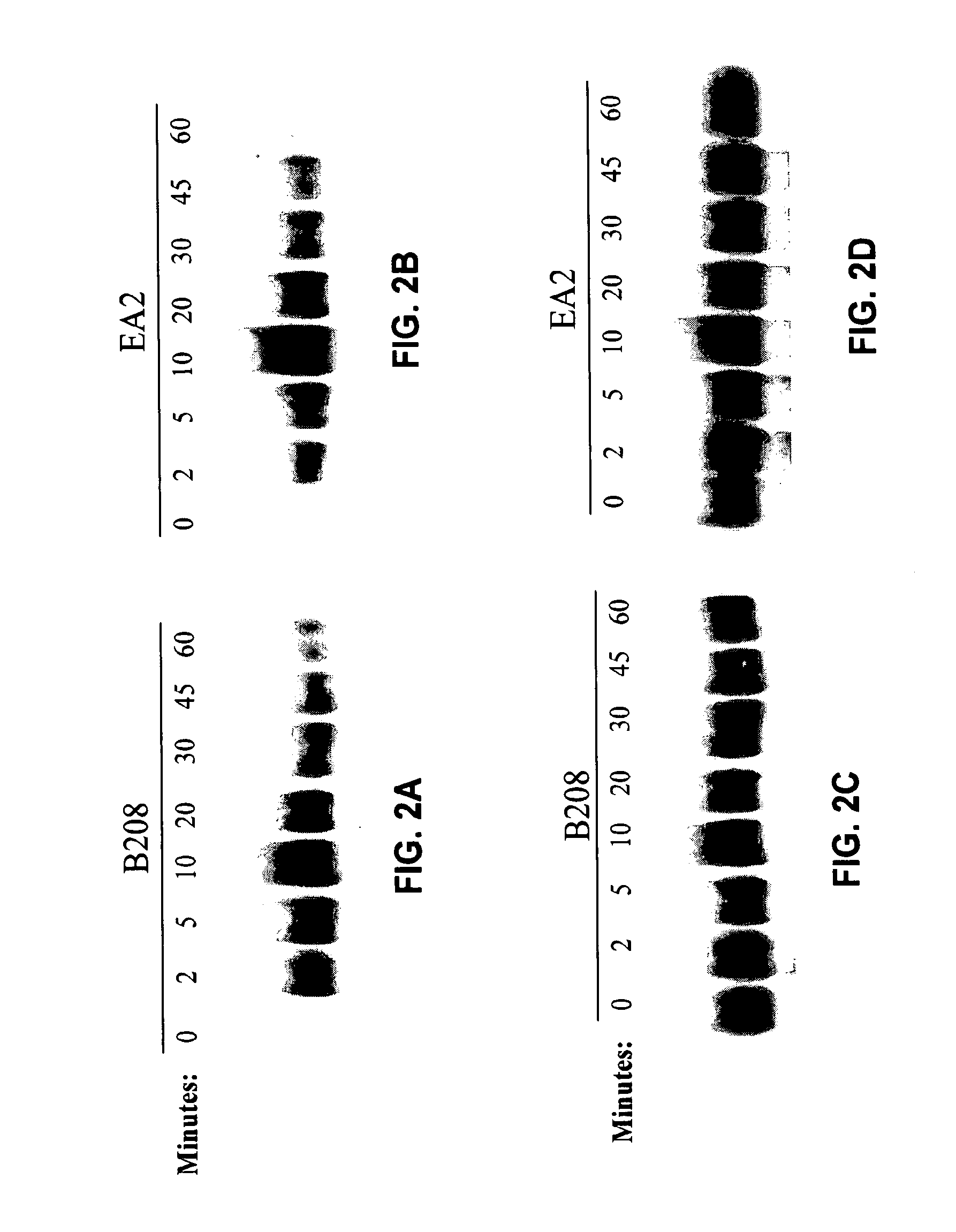
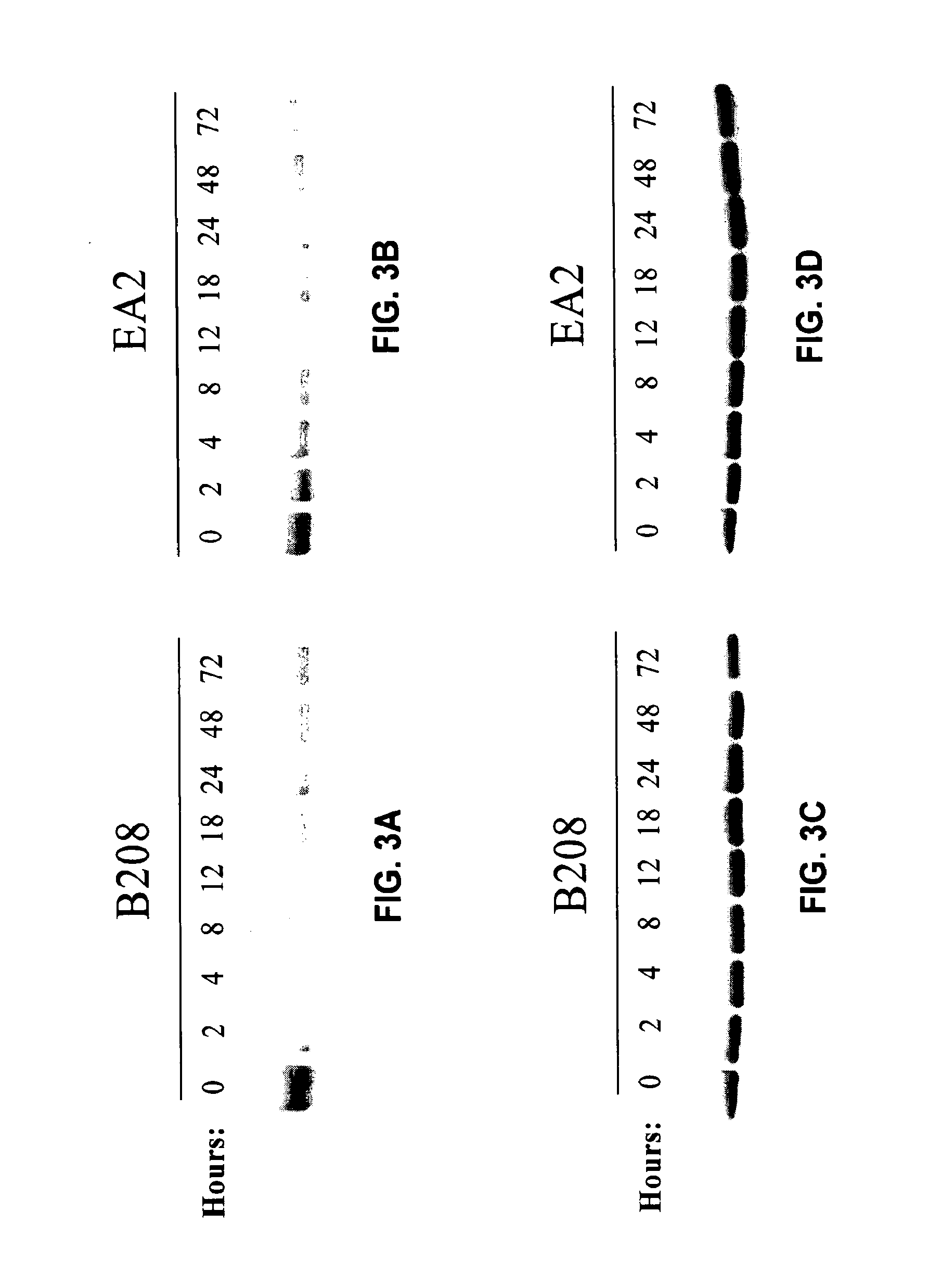
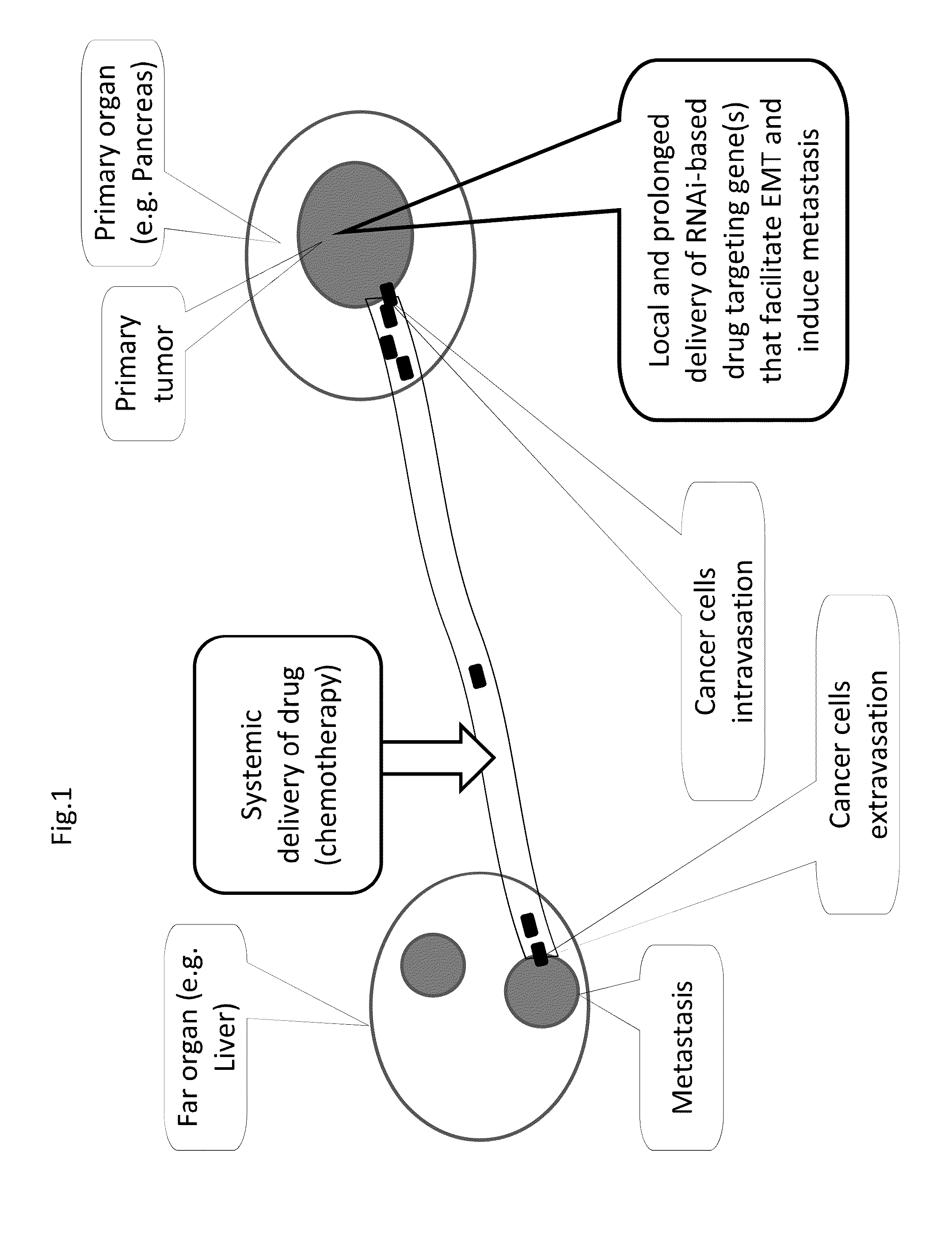
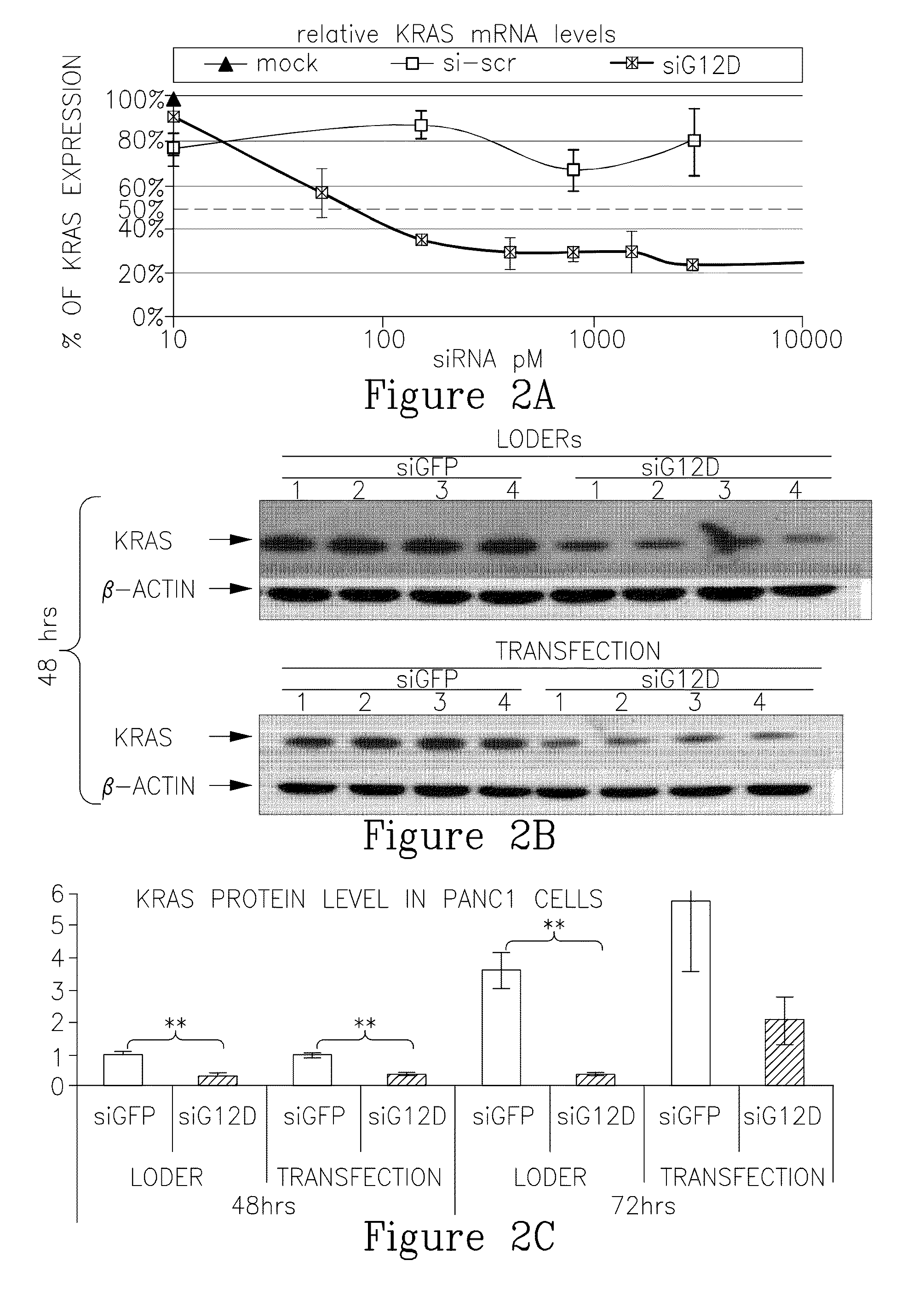
METHODS AND COMPOSITIONS FOR RNAi-BASED CANCER TREATMENT
InactiveUS20140314854A1Organic active ingredientsPharmaceutical non-active ingredientsPrimary tumorNucleotide
The present invention generally concerns methods and nucleotide-based compositions for treating metastatic cancer that is associated with a primary tumor or a few tumors at the primary organ.
Owner:SILENSEED LTD
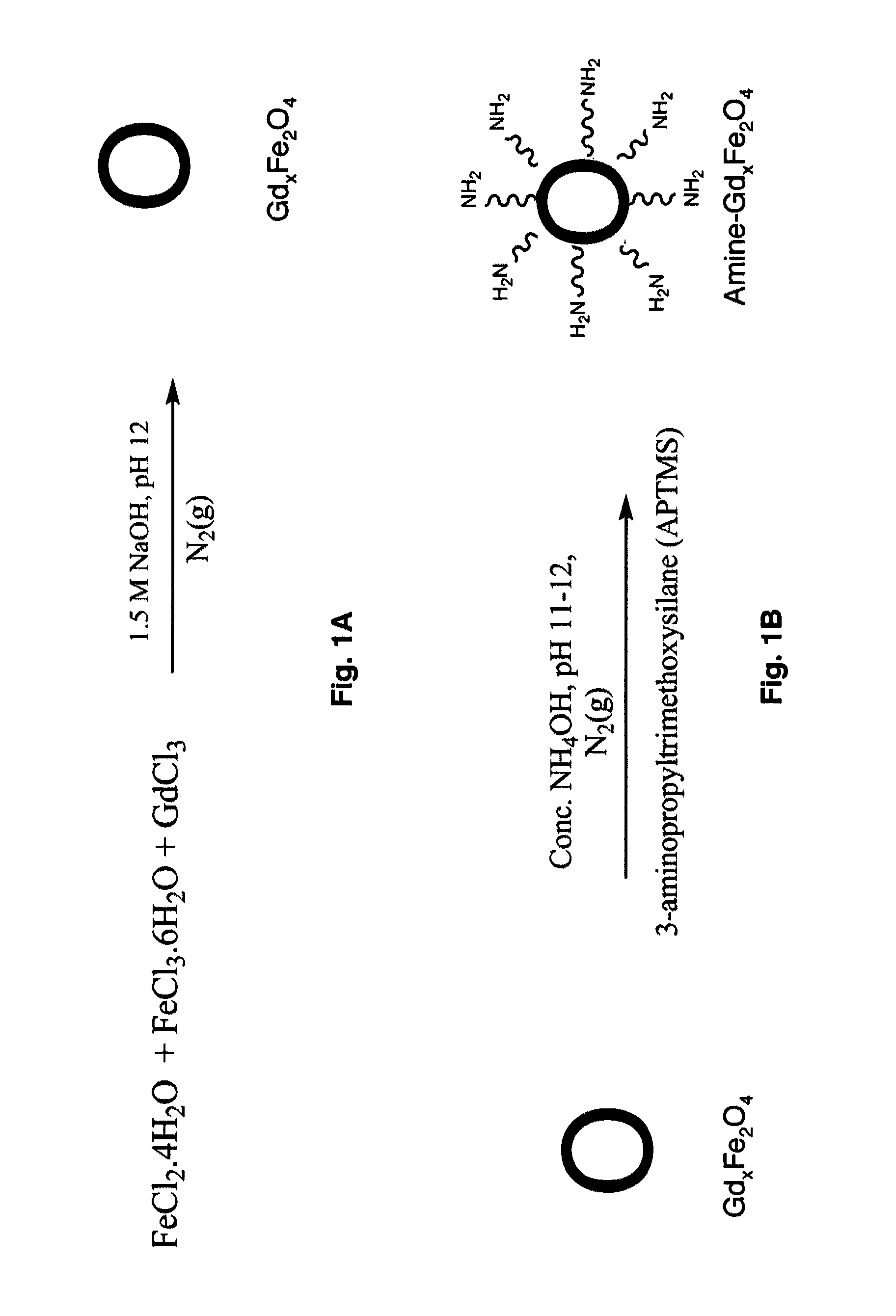
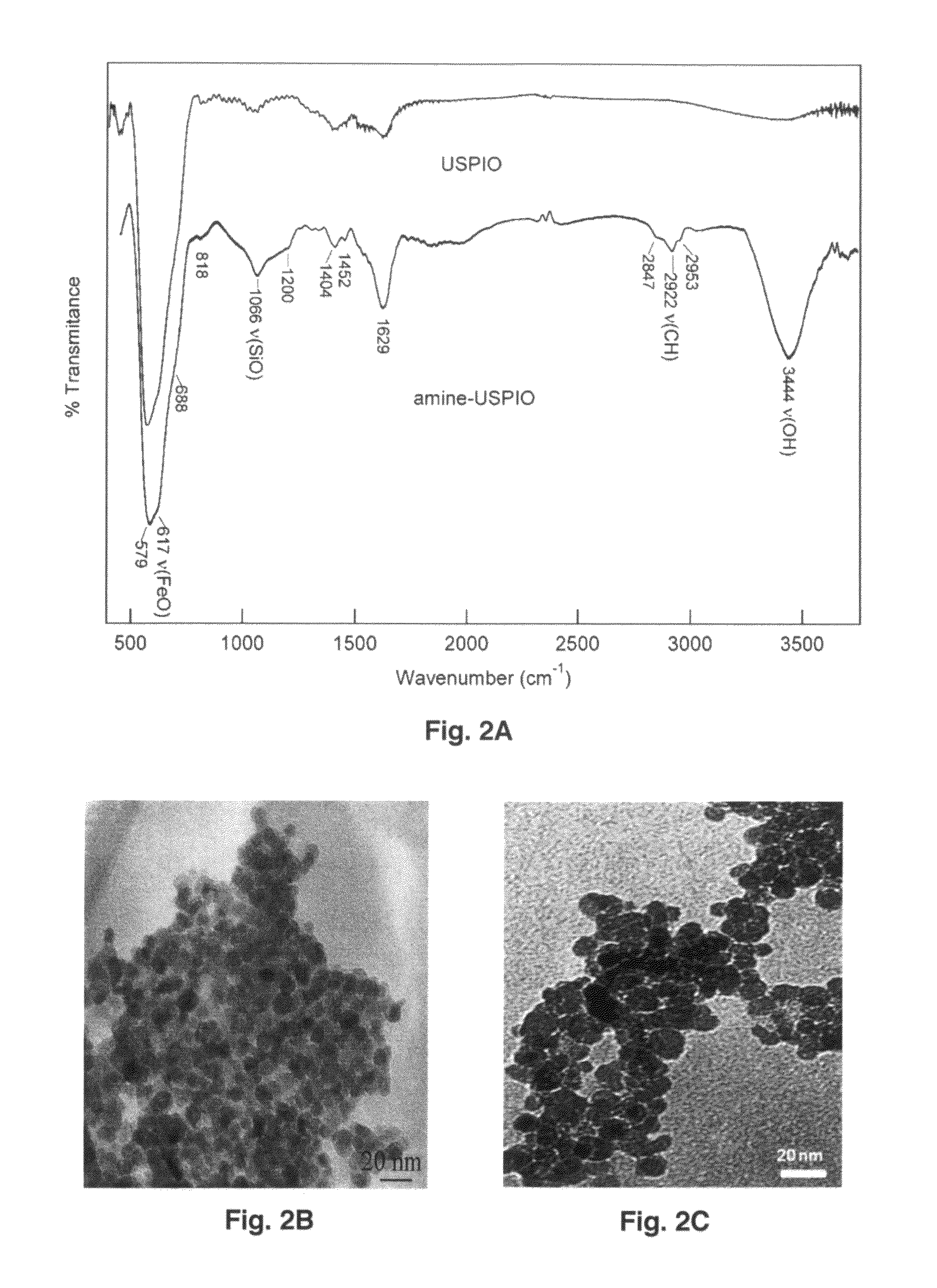
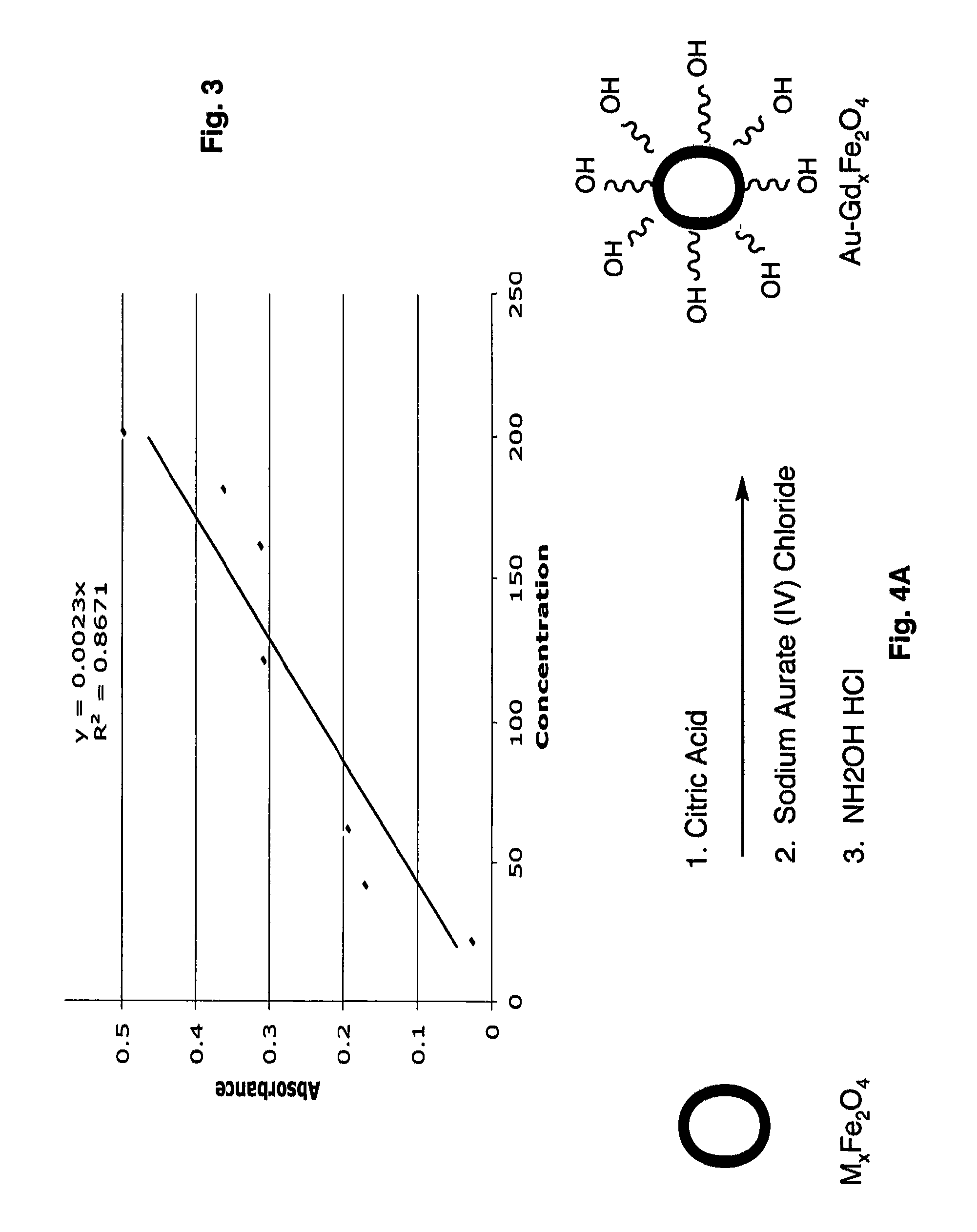
Ultrasmall superparamagnetic iron oxide nanoparticles and uses thereof
The present invention provides biomimetic contrast agents, dual functional contrast agents effective for therapeutic gene delivery and magnetic nanoparticles which comprise functionalized iron oxide nanoparticle cores, one of an inert gold layer, a layer of inert metal seeds or a silica layer and, optionally, one or both of an outer gold-silver nanoshell or a targeting ligand attached to the inert gold layer or the gold-silver nanoshell. Also provided are methods of in vivo magnetic resonance imaging, of treating primary or metastatic cancers or of ablating atherosclerotic plaque using the contrast agents and magnetic particles. In addition, kits comprising the biomimetic contrast agents, dual contrast agents and magnetic nanoparticles.
Owner:HOUSTON SYST UNIV OF
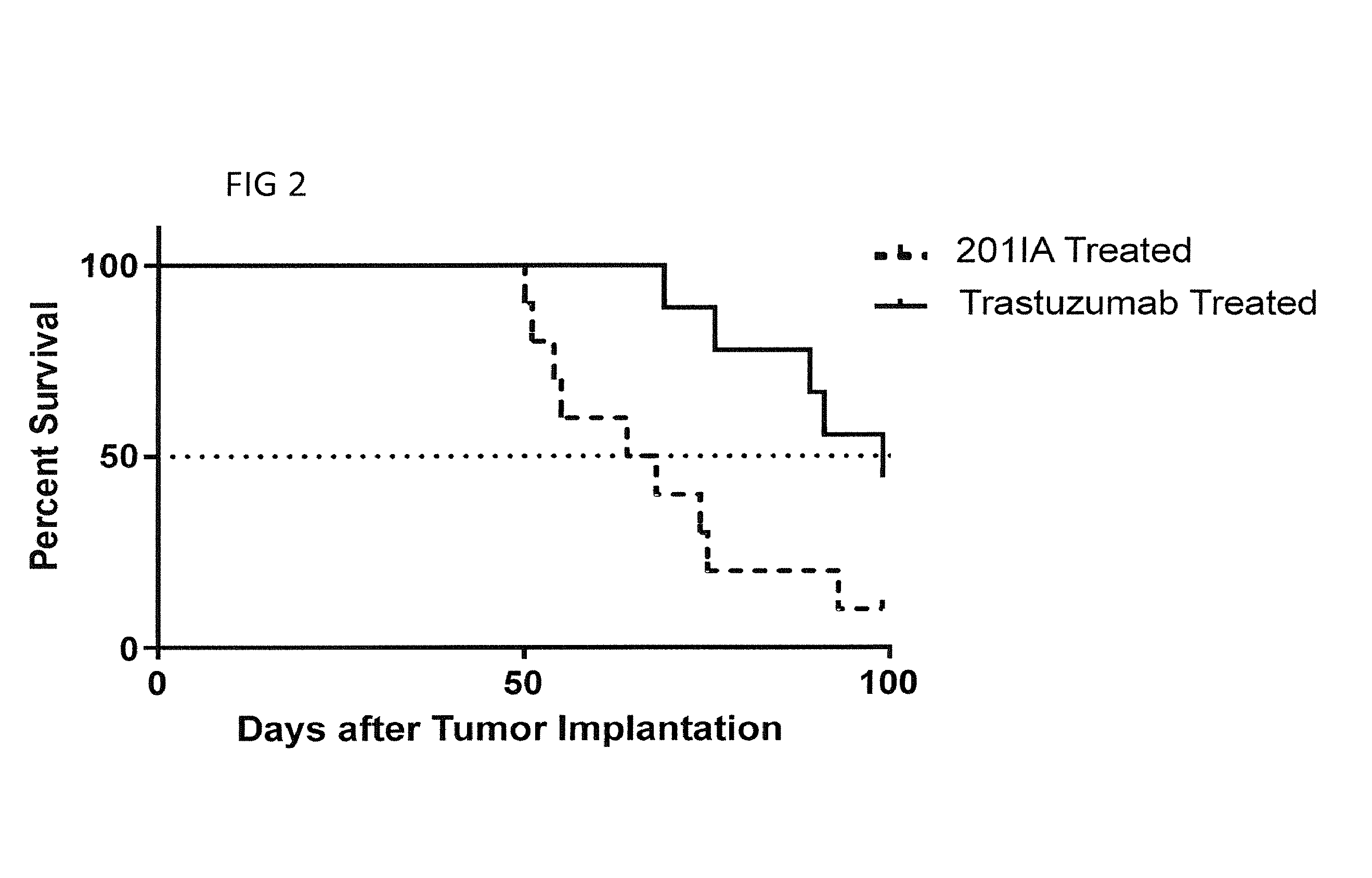
Use of entrained neutrophils to treat metastatic and micrometastatic disease in at risk patients
The present invention relates generally to compositions and methods for treating cancer patients with a poor prognosis, and to therapeutic modalities for improving prognosis by combating metastasis and abrogating chemoresistance in cancer cells. In particular, the invention relates to the role of white blood cells, i.e. neutrophils and neutrophil-like cells, in preventing the spread of cancer from a primary tumor to secondary locations in the body. The invention provides methods for reducing or delaying the spread of metastatic cancer cells in a patient at risk for metastatic tumor development, at risk for metastatic relapse, i.e. prophylactic methods, and treating patients suffering from metastatic tumors.
Owner:MEMORIAL SLOAN KETTERING CANCER CENT
Recombinant anti-PD-L1 monoclonal antibody
PendingCN110878122AHigh affinityGood antitumor effectImmunoglobulins against cell receptors/antigens/surface-determinantsAntibody ingredientsComplementarity determining regionAntiendomysial antibodies
The invention discloses a recombinant anti-PD-L1 monoclonal antibody, relates to the technical field of biological medicines, and is used for providing an effective treatment medicine for patients with advanced or metastatic cancers, in particular to the patients with ineffective or drug-resistant treatment by an existing anti-PD-L1 medicine. The complementarity determining region of the antibodyhas sequences shown in SEQ ID NO: 1 to SEQ ID NO: 6. Compared with an existing anti-PD-L1 drug, the antibody has a unique binding epitope, and is better in affinity to human PD-L1 and better in tumorinhibition effect.
Owner:SHANGHAI ZHANGJIANG BIOTECH
Novel Small-Molecule Inhibitors of Rac1 in Metastatic Breast Cancer
A novel inhibitor of Rac activity based on the structure of the established Rac / Rac-GEF inhibitor NSC23766 is discloses. The compound EHop-016, with an IC50 of 1.1 μM, is a 100-fold more efficient inhibitor of Rac activity than NSC23766. EHop-016 is specific for Rac1 and Rac3 at concentrations ≦5 mM. At higher concentrations, EHop-016 inhibits the close homolog Cdc42. In MDA-MB-435 cells, EHop-016 (≦5 mM) inhibits the association of the Rac-GEF Vav2 with a nucleotide-free Rac1(G15A), which has a high affinity for activated GEFs. EHop-016 does not affect the association of the Rac-GEF Tiam-1 with Rac1(G15A) at similar concentrations. EHop-016 also inhibits the Rac activity of MDA-MB-231 metastatic breast cancer cells and reduces Rac-directed lamellipodia formation in both cell lines. EHop-016 decreases Rac-downstream effects of p21-activated kinase (PAK)1 activity and directed migration of metastatic cancer cells. At low concentrations (<5 μM), EHop-016 does not affect cell viability.
Owner:UNIVERSITY OF PUERTO RICO
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com