Recombinant anti-PD-L1 monoclonal antibody
A monoclonal antibody, PD-L1 technology, applied in the field of biomedicine, can solve the problem of no obvious response and achieve high affinity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
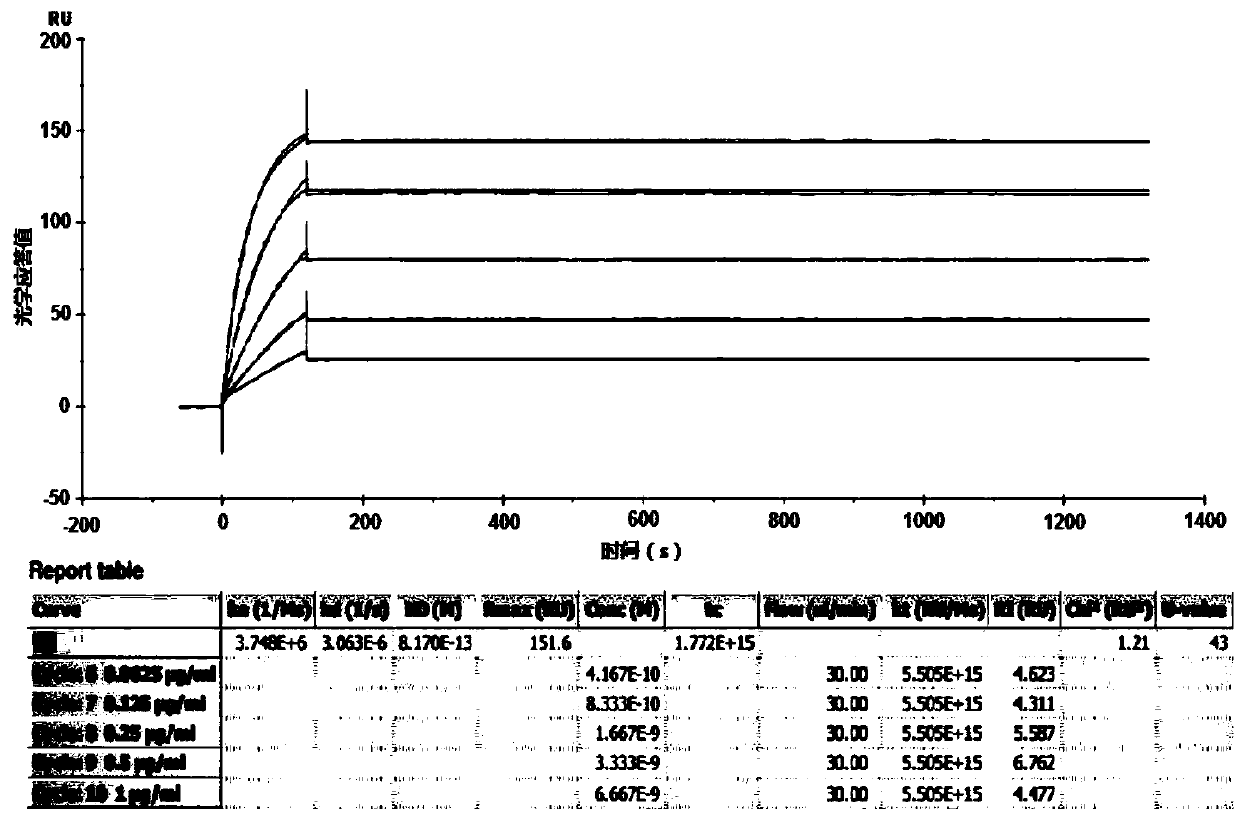
[0060] Embodiment 1, construction and screening of hybridoma cells
[0061] 1. Immunized animals
[0062] Use the recombinantly expressed PD-L1-Fc fusion protein (fused from the extracellular region of human PD-L1 to the Fc segment of the antibody, the amino acid sequence is the sequence numbered Q9NZQ7-1 on the UniProt website) with a dose of 100-500 μg / mL intraperitoneally Immunize Balb / c mice for 3 times. 3-7 days before cell fusion, boost immunization with PD-L1-Fc fusion protein 50-100 μg / mL tail vein.
[0063] 2. Cell Fusion
[0064] The mouse myeloma cell line NS-1 was fused with the splenocytes of Balb / c mice after immunization, and then placed in a 96 empty cell culture plate, screened with a complete medium containing HAT, half of the medium was changed in 3-5 days, 2 Colony formation can be seen in about a week.
[0065] 3. Identification of culture supernatant
[0066] The hybridoma cell culture supernatant was detected by ELISA technique. Coat multi-well pla...
Embodiment 2
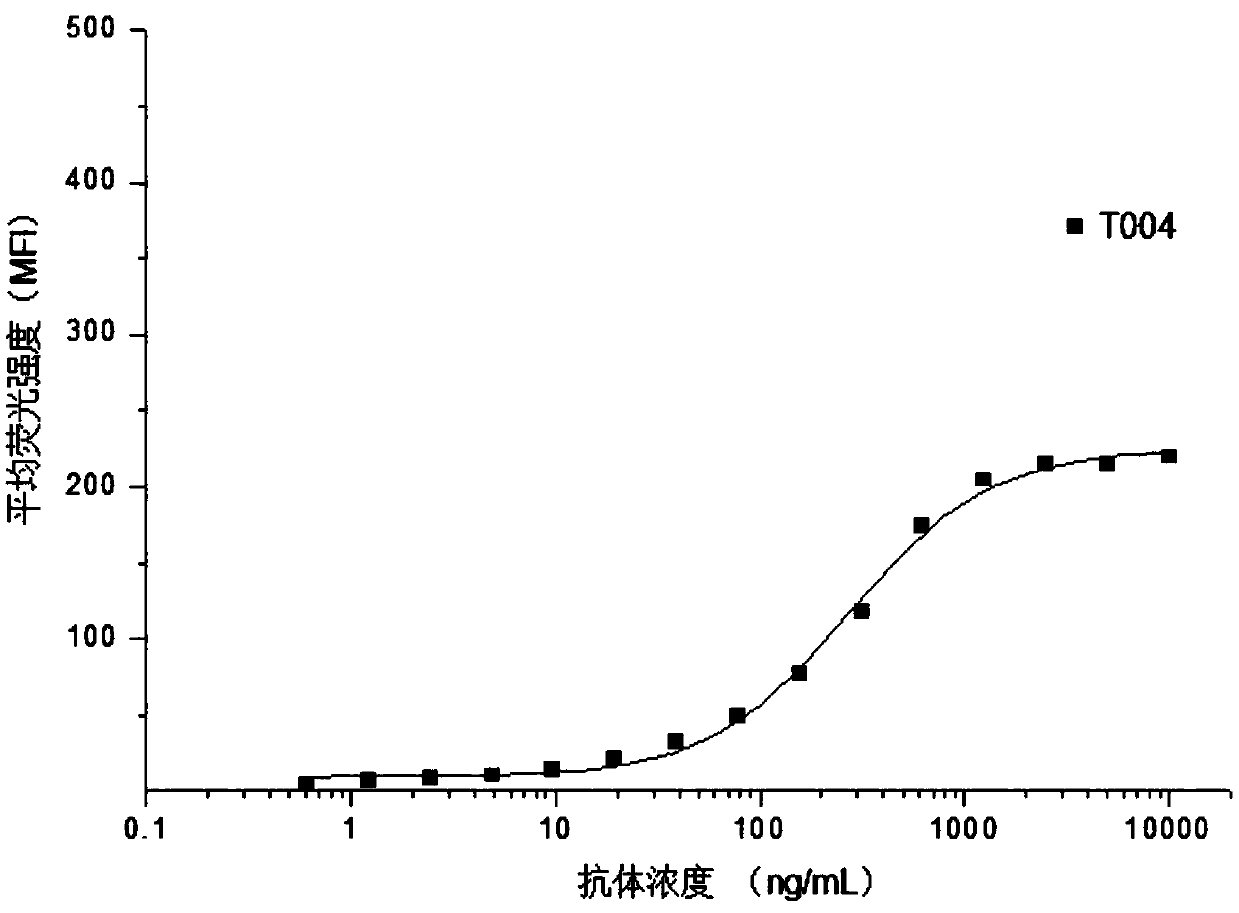
[0071] Example 2. Flow cytometry detection of the binding ability of T0004 antibody to PD-L1 on the cell membrane surface
[0072] The expression vector plasmid containing the full-length coding sequence of human PD-L1 was stably transfected into CHO-K1 host cells, and CHO-K1 engineered cells stably expressing PD-L1 on the membrane surface were obtained by pressurized screening (denoted as CHO-K1 / hmPD- L1). T0004 antibody was diluted to 15 concentrations starting from 10 μg / mL, mixed with CHO-K1 / hmPD-L1 cells in the logarithmic growth phase, and kept on ice for 45 min to make it adhere to the surface of CHO-K1 / hmPD-L1 cells PD-L1 binding. Wash 2 times with PBS containing 1% fetal bovine serum, add FITC fluorescently labeled rabbit anti-mouse IgG (H+L) secondary antibody, ice bath for 45min, wash 2 times with PBS containing 1% fetal bovine serum. The pellet was resuspended in PBS containing 1% fetal bovine serum, and the fluorescence intensity was analyzed by flow cytometry. ...
Embodiment 3
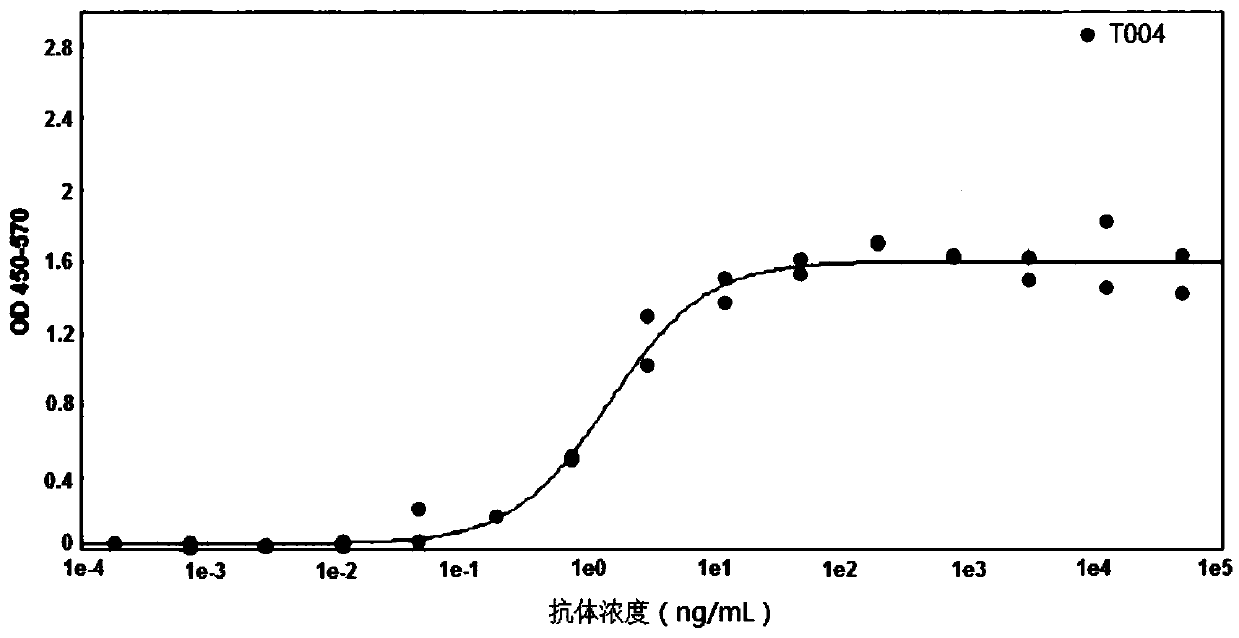
[0074] Example 3. Enzyme-linked immunoassay detection of binding ability of T0004 antibody to soluble PD-L1
[0075] Dilute the human PD-L1-Fc fusion protein to 10 μg / mL with coating solution, add 100 μL / well to the microtiter plate, and coat at 37°C for 1-2 hours. Discard the coating solution, add blocking solution, 350 μL / well, block overnight at 2-8°C, and wash 7 times with a plate washer. Dilute the T0004 antibody with PBS, starting from 50000ng / mL, dilute 15 concentrations at a 4-fold ratio, 100μL / well, react at 37°C for 1-2 hours, discard the liquid in the microplate plate, and wash 7 times with a plate washer. Add horseradish peroxidase (HRP)-labeled goat anti-mouse IgG (H+L) diluted 1:10000, 100 μL / well, react at 37°C for 1 hour, discard the liquid in the microtiter plate, and wash 7 times with a plate washer . Add TMB substrate solution to the ELISA plate, 50 μL / well, develop color in the dark for 1-10 minutes, add 50 μL / well stop solution, and mix quickly. Read OD...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com