METHODS AND COMPOSITIONS FOR RNAi-BASED CANCER TREATMENT
a cancer treatment and composition technology, applied in the field of nucleotide-based agent-based and rnai-based cancer treatment methods and compositions, can solve the problems of slow pc disease metastasis and epithelial-to-mesenchymal transition, and achieve the effect of reducing the levels of downstream effector proteins
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Schematic Depiction of the Described Treatment
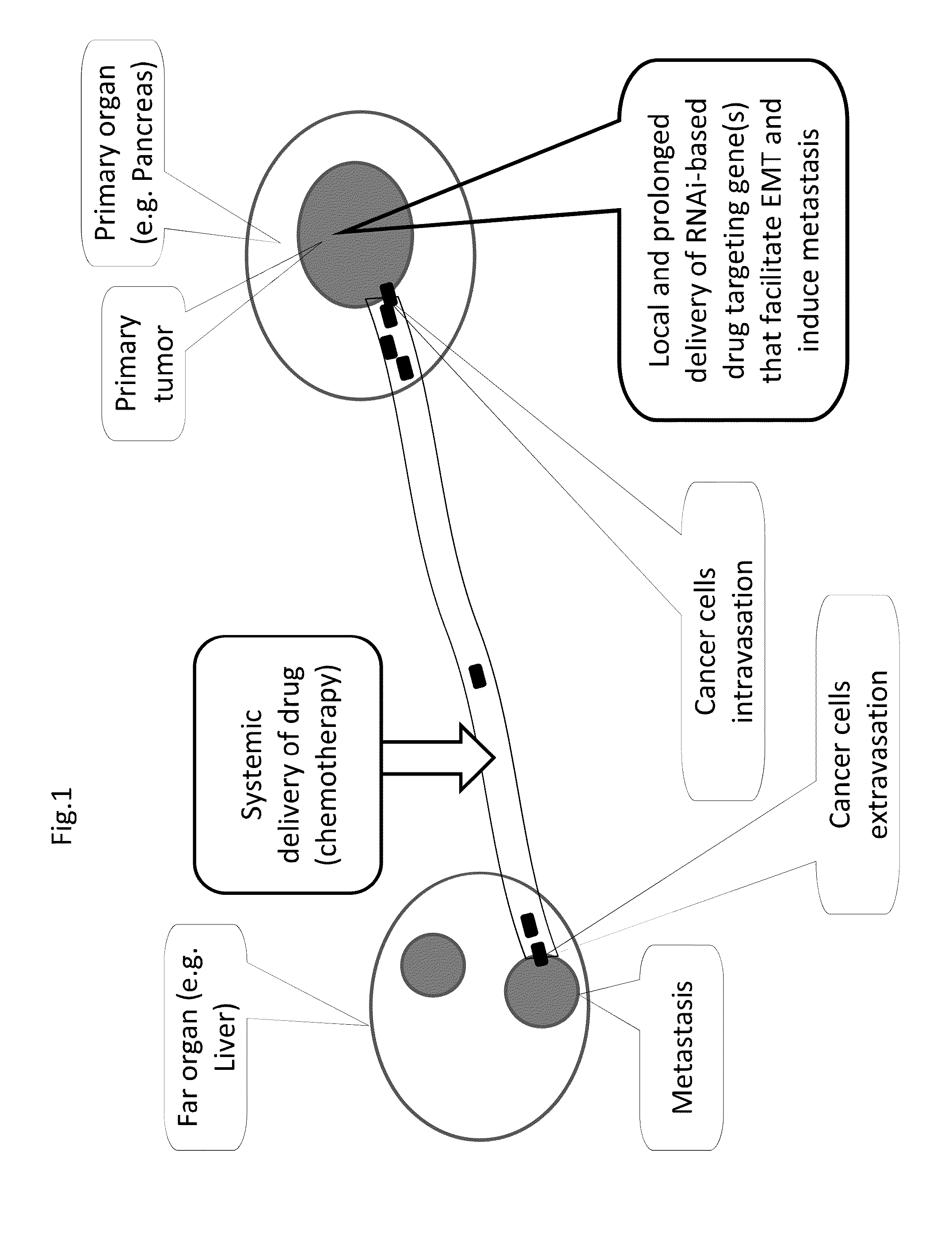
[0160]FIG. 1 shows a schematic depiction of the described treatment of metastatic cancer. Local and prolonged delivery of RNAi-based treatment within a primary tumor is administered, often in parallel to systemic administration of drugs including chemotherapy drugs
example 2
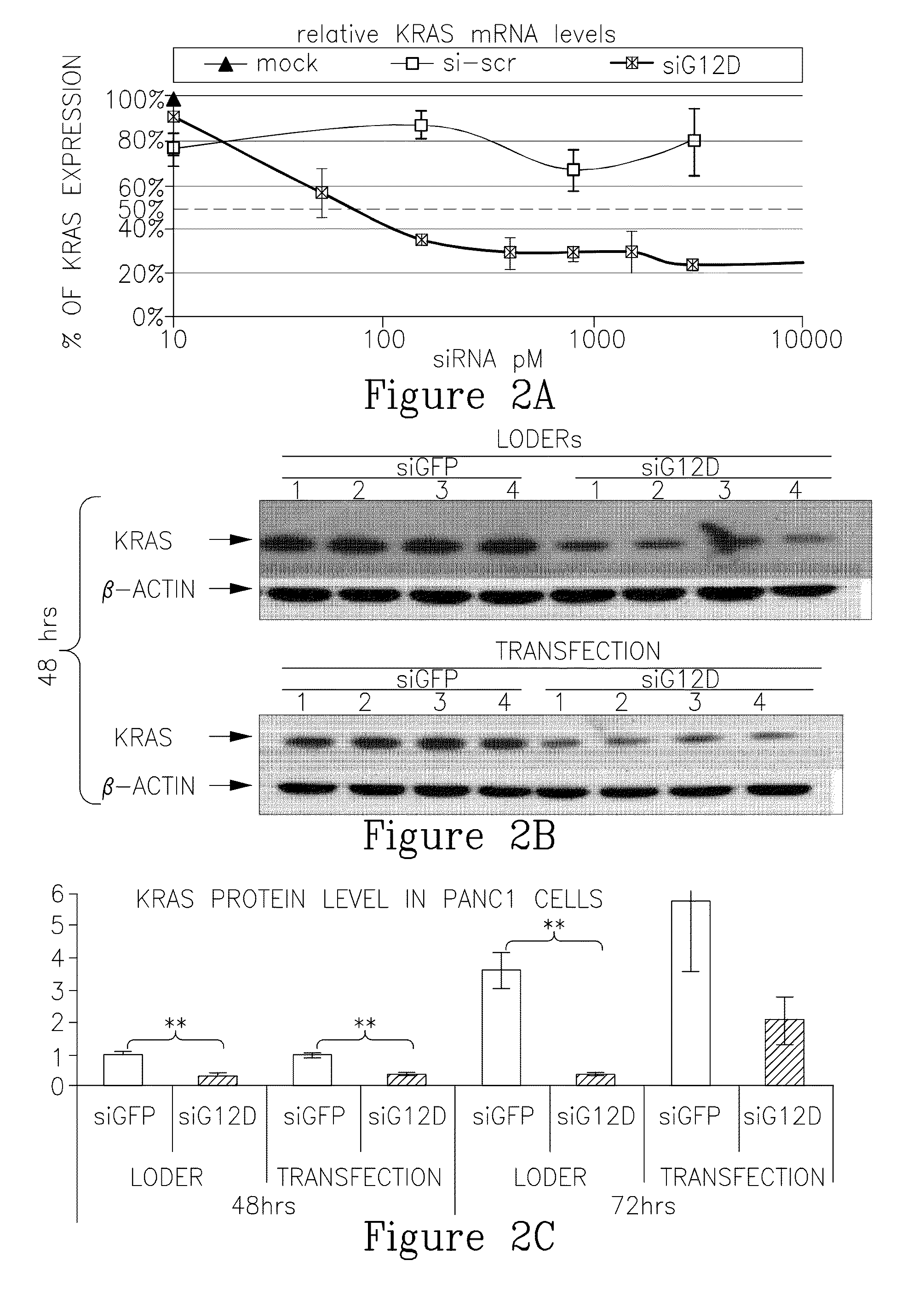
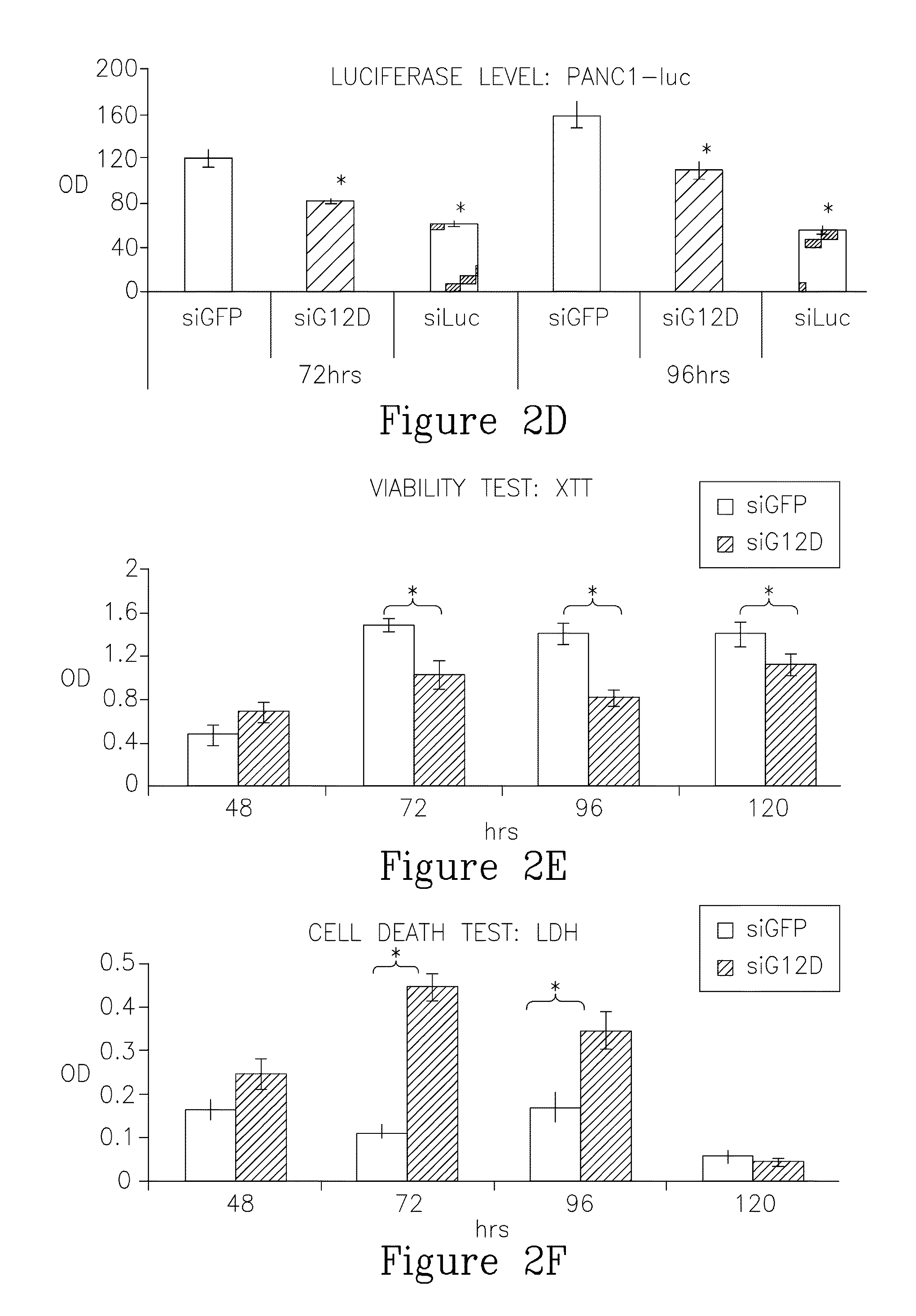
LODER-Derived siG12D Significantly Inhibits Growth of Pancreatic Cancer Cells In Vitro
Methods
[0161]DDD's containing RNAi molecules were produced in a biological-class hood in a clean room, as follows:
[0162]Step 1: Preparation of siRNA / D-Mannitol / Sodium Bicarbonate Mixture:
[0163]siRNA was added to the pre-weighed D-Mannitol and Sodium Bicarbonate, and they were dissolved in RNase-free sterile water.
[0164]Step 2: Freezing:
[0165]The liquid was placed into glass vials, frozen in dry ice, and lyophilized for 48 hours.
[0166]Step 3: PLGA Preparation:
[0167]PLGA was dissolved in Ethyl Acetate.
[0168]Step 4: Combining PLGA with D-Mannitol / Sodium Bicarbonate / siRNA:
[0169]The PLGA solution was poured into the glass vial containing the lyophilized D-Mannitol / Sodium Bicarbonate / siRNA in fractions and stirred until homogenization
[0170]Step 5: Solvent Evaporation.
[0171]The solution was poured into a Teflon-covered dedicated glass dish and left to evaporate inside a dedicated container for 3-5 days.
[0...
example 3
Functionality of the siRNA LODER In Vivo
[0181]For the in vivo assessment of LODER-siRNA effects, we initially used the murine colon cancer CT26 cells. These cells were stably transfected to constitutively express the luciferase gene (CT26-LUC cells). CT26-LUC cells were injected subcutaneously into BALB / c mice. When tumor volume reached ˜1 cm3, two siLuc or control (siGFP) LODERs were implanted into the tumors. As seen in FIG. 3A, luciferase expression increased by approximately 3-fold in the siGFP LODER-implanted mice, while in mice implanted with the siLuc LODERs, the luciferase levels barely increased. FIG. 3B reveals that there was no significant effect on tumor weight, at sacrifice, in the group treated with the siLuc LODER in comparison with the siGFP LODER group, indicating that the lower Luciferase levels were not caused by an antitumor effect; rather, the siLuc released from the LODER inhibited luciferase expression, as measured by its activity in vivo.
[0182]We also assesse...
PUM
| Property | Measurement | Unit |
|---|---|---|
| time | aaaaa | aaaaa |
| hydrophobic-hydrophilic interactions | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com