A detecting method for improving tiopronin active pharmaceutical ingredient stability
A tiopronin and detection method technology, which is applied in the field of detection to improve the stability of tiopronin API, can solve problems such as poor system applicability, unreasonable impurity control limits, and inability to separate adjacent impurities, so as to solve the problem of measurement technology , to ensure the effect of safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
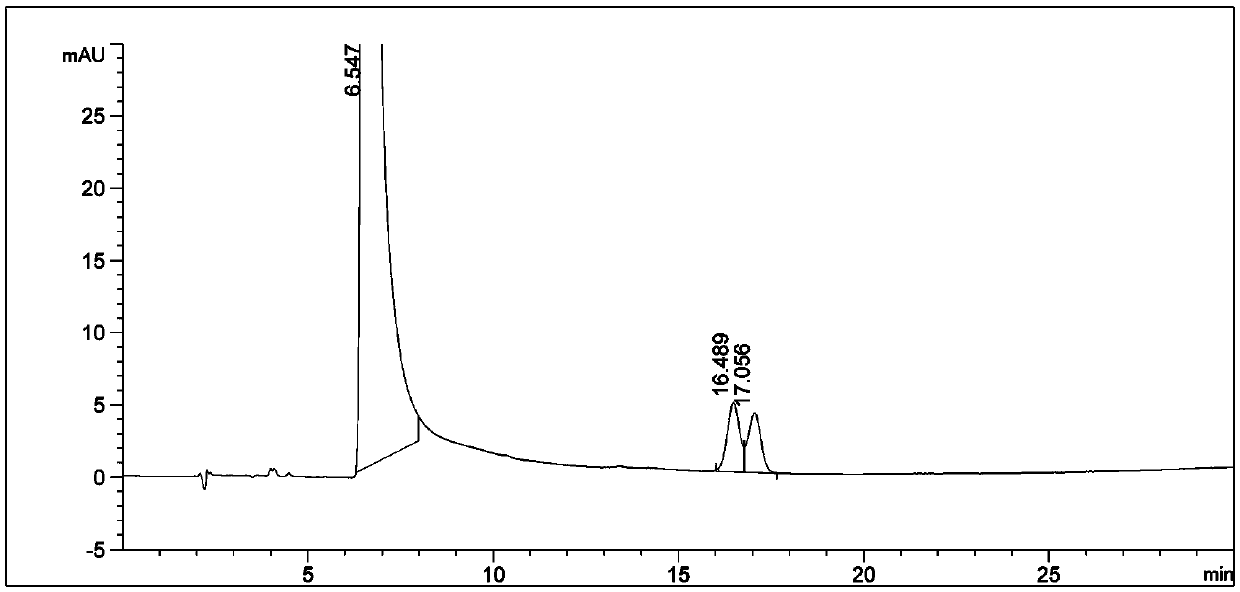
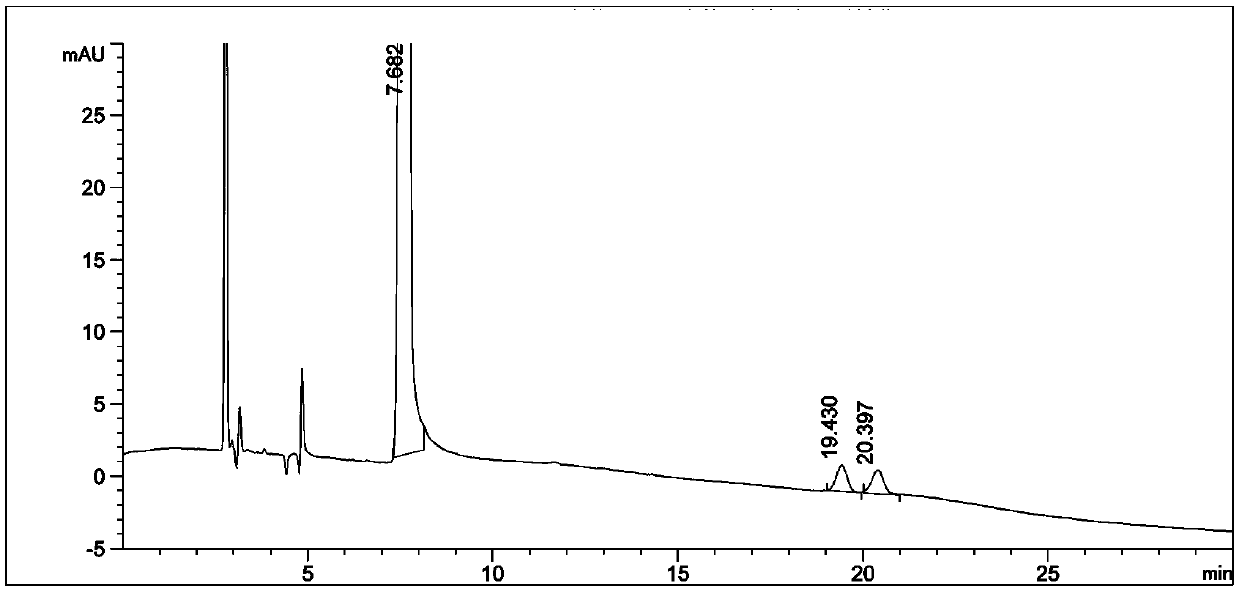
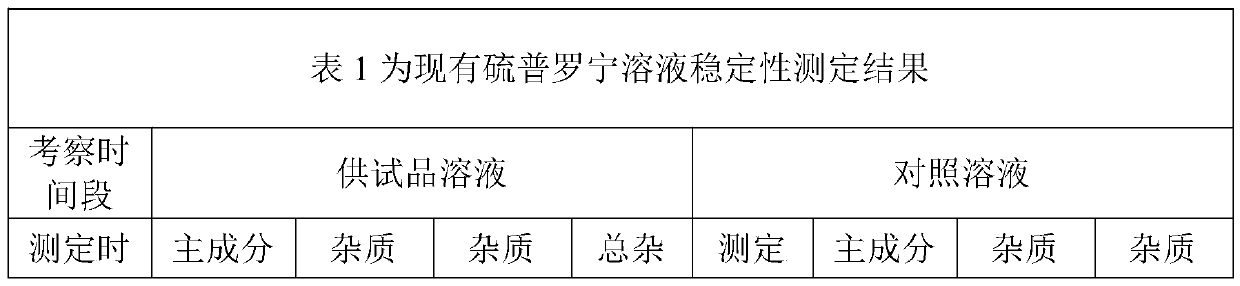
[0026] Through the strong degradation test of this product, it is found that the main degradation route is oxidative degradation under humid heat conditions. The conclusion is shown in Chart 2:
[0027] Specific strong degradation test operation:
[0028] Take by weighing the bulk drug 50g of tiopronin batch A in a 50ml measuring bottle, add a solvent to dissolve and dilute to the mark, as the standard solution of the bulk drug.
[0029] In addition, take the bulk drug that adopts tiopronin batch A to produce a batch of tablets according to the company's prescription, take 20 tablets, peel off the coating, take the tablet core and grind it finely, and take 160mg of fine powder in a 50ml measuring bottle , dissolve with a solvent and dilute to the mark as a standard solution for tablets.
[0030] Weigh 50 g of the bulk drug of tiopronin batch A, add 2 ml of 0.1 mol / L hydrochloric acid solution to a 50 ml measuring bottle, leave it at room temperature for 4 hours, add solvent a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com