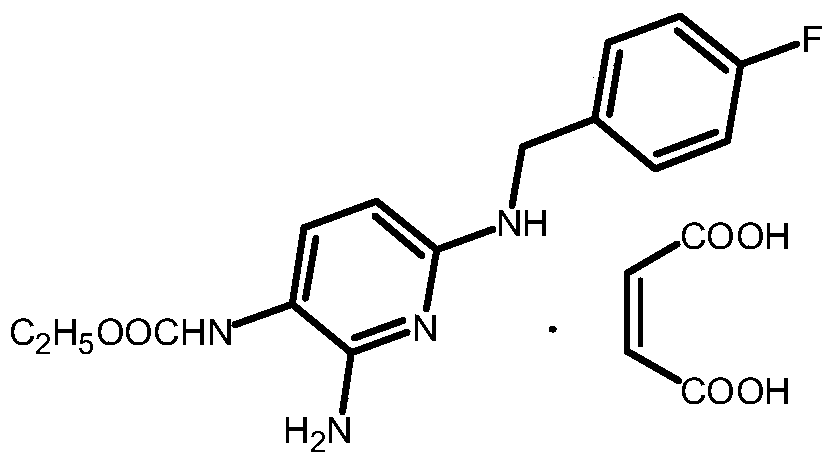
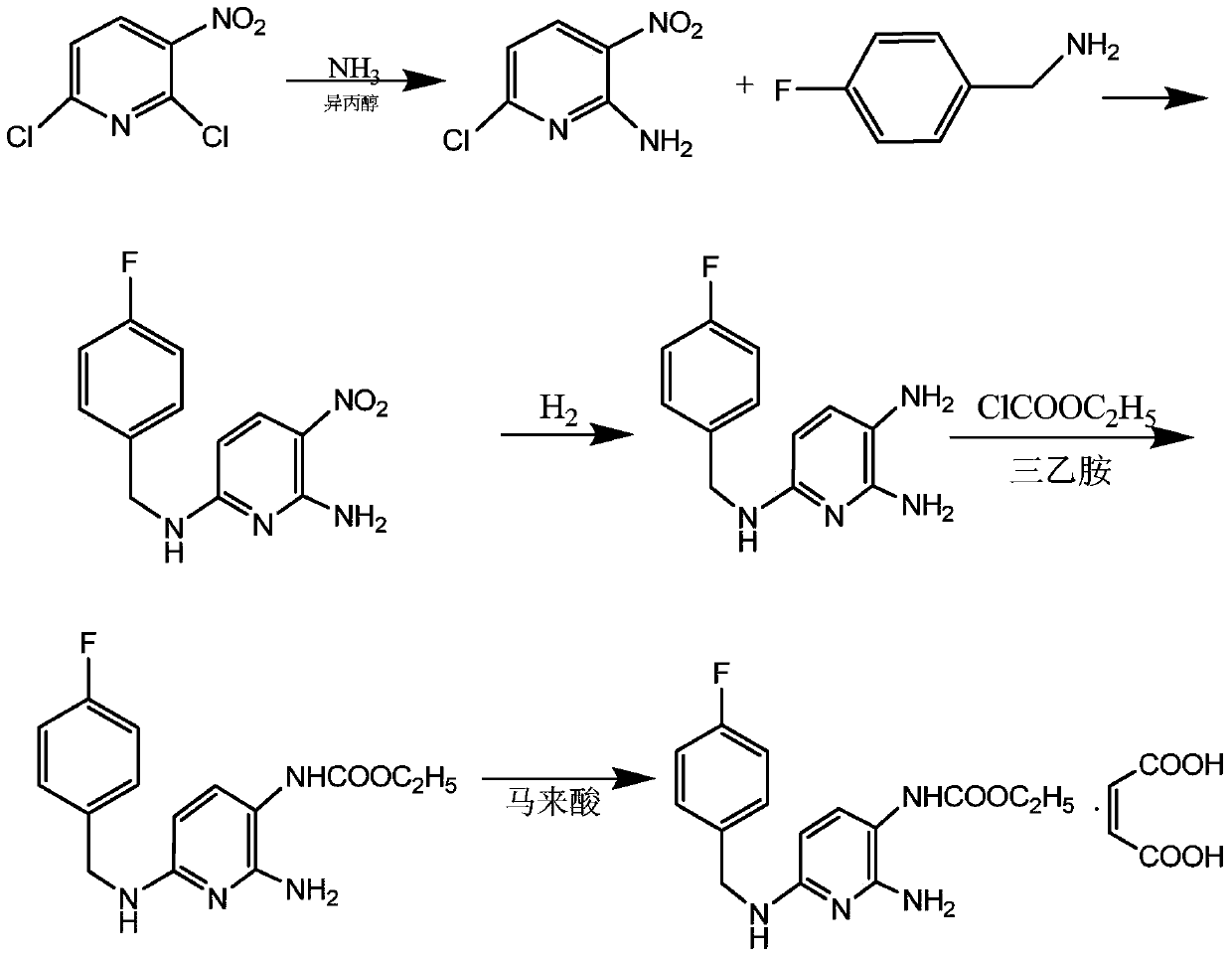
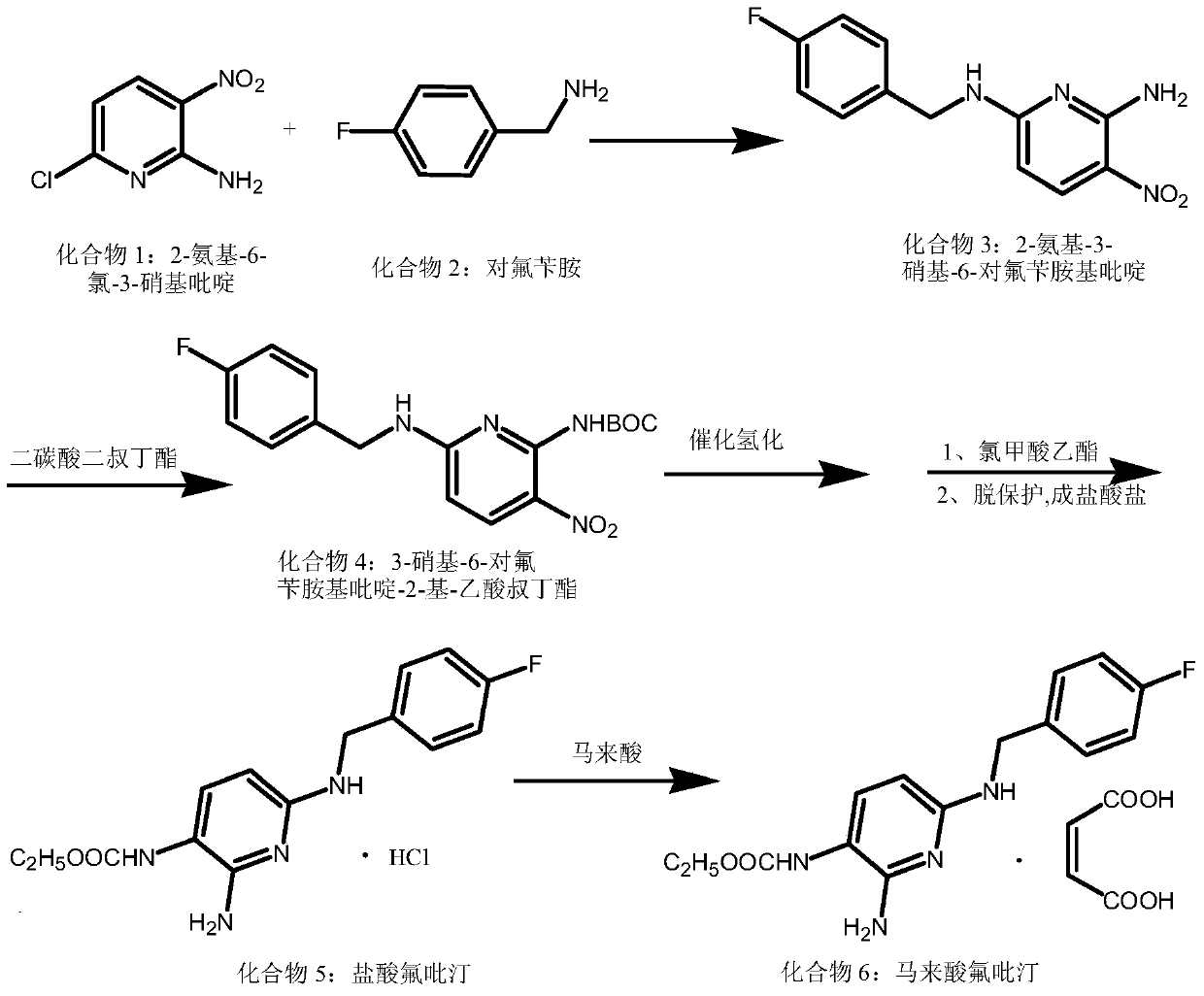
A kind of synthetic method of flupirtine maleate compound
A technology of flupirtine maleate and a synthesis method, applied in the field of medicine and chemical industry, can solve the problems of harmful environment, unstable product quality and high production cost, and achieve the effects of improving product yield, reducing product purity and easy reaction process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Example 1: Preparation of 2-amino-3-nitro-6-p-fluorobenzylaminopyridine (compound 3)
[0028] Add 200mL of methanol, 52.08g of compound 1, 37.54g of p-fluorobenzylamine, and 50.0mL of triethylamine into the reaction bottle, heat up to 80°C and react for 10 hours. After the reaction is completed, add the reaction solution to 10 times of water, precipitate a solid, and dry it by suction filtration. 76.47 g of compound 3 was obtained with a yield of 97.2% and a purity of 99.62% (HPLC).
Embodiment 2
[0029] Example 2: Preparation of 3-nitro-6-p-fluorobenzylaminopyridin-2-yl-acetic acid tert-butyl ester (compound 4)
[0030] Suspend 26.22g of compound 3 (100mmol) in 50ml of dry DMF, add 12.0g of sodium hydroxide at room temperature to obtain a brown solution, add 34.50ml of Boc 2 O (150mmol), after stirring for 10 minutes, the mixture was heated to 40°C overnight, the reaction mixture was poured into water, extracted with ethyl acetate (2×100mL), the aqueous phase was adjusted to pH 4-5 with 2M HCl aqueous solution, and Chloromethane (3×100 mL) was extracted, and the combined organic phases were concentrated to dryness under reduced pressure to obtain 24.27 g of compound 4 with a yield of 97.0% and a purity of 99.7% (HPLC).
Embodiment 3
[0031] Example 3: Preparation of 3-nitro-6-p-fluorobenzylaminopyridin-2-yl-acetic acid tert-butyl ester (compound 4)
[0032] Suspend 26.22g (100mmol) of compound 3 in 50ml of dry dioxane and water, add 41.73ml of triethylamine dropwise at room temperature to obtain a brown solution, add 23.00ml of Boc 2 O (100mmol) was stirred for 10 minutes, the mixture was heated to 50°C overnight, the reaction mixture was poured into water, extracted with ethyl acetate (2×100mL), the aqueous phase was adjusted to pH 4-5 with 2M HCl aqueous solution, and dichloro Methane (3×100 mL) was extracted, and the combined organic phases were concentrated to dryness under reduced pressure to obtain 23.77 g of compound 4 with a yield of 95.0% and a purity of 99.7% (HPLC).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com