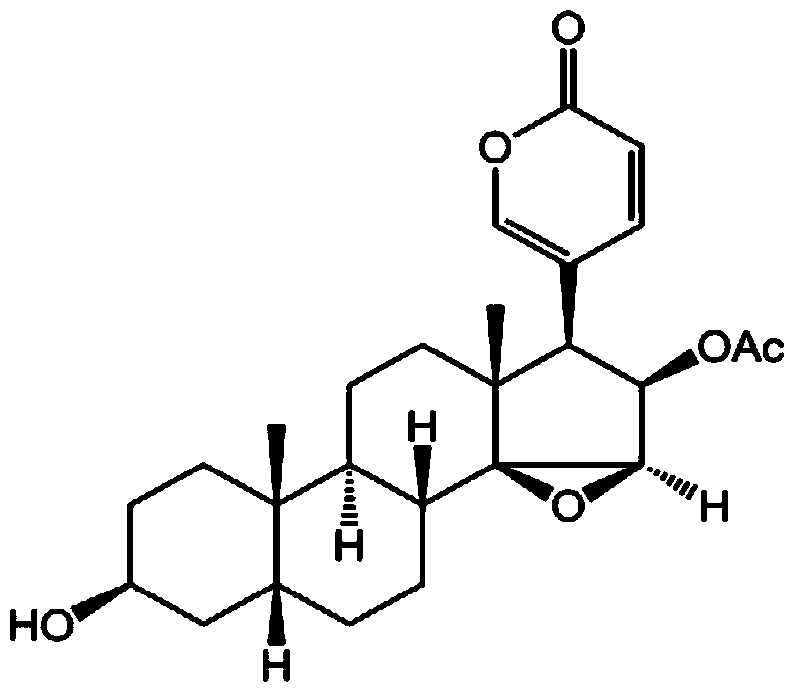
Novel use of cinobufagin and resibufogenin in inhibition of enterovirus type 71 infection
A technology of cinobufafonitoxin and esterbufagenin, which can be used in antiviral agents, medical preparations containing active ingredients, and pharmaceutical formulas, and can solve problems such as antiviral effects that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Construction of RDS cells
[0035] The synthetic EV71 / CA16 receptor hSCARB2 gene fragment (NCBI accession number: NM_005506.3, synthesized by Shanghai Jierui Company) with EcoRI&XbaI restriction sites at both ends was inserted into the lentiviral vector Lenti-XpLVX-Puro vector (purchased from Clontech ), and use the transfection reagent in the lentiviral vector kit (purchased from Clontech) to transfect human rhabdomyosarcoma cells (RD cells, purchased from ATCC, accession number #CCL-136) according to the instructions to obtain RD stably transfected with hSCARB2 cells, referred to as RDS cells (for the specific construction method of RDS cells, refer to Lietal. Virology Journal, 10:250).
Embodiment 2
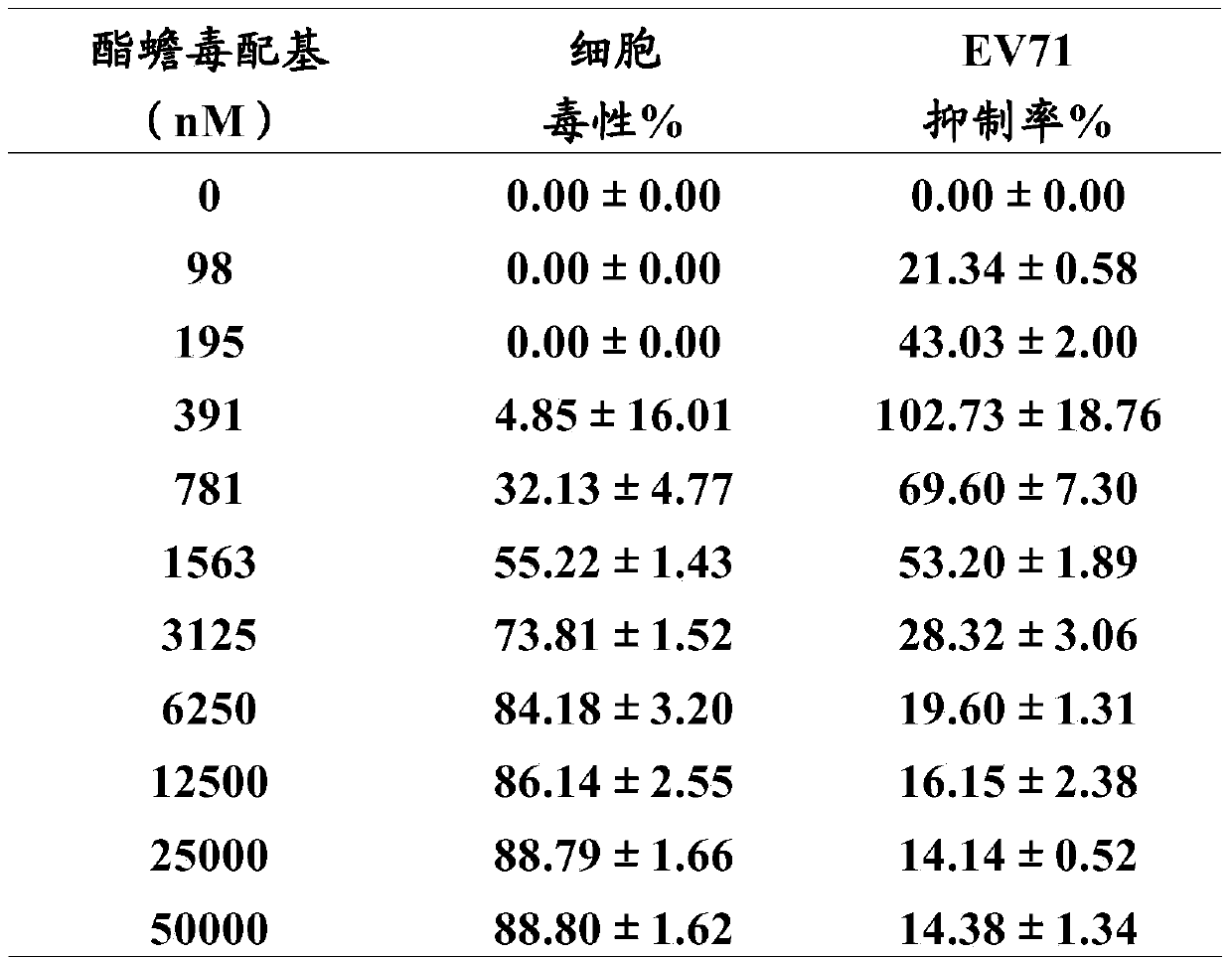
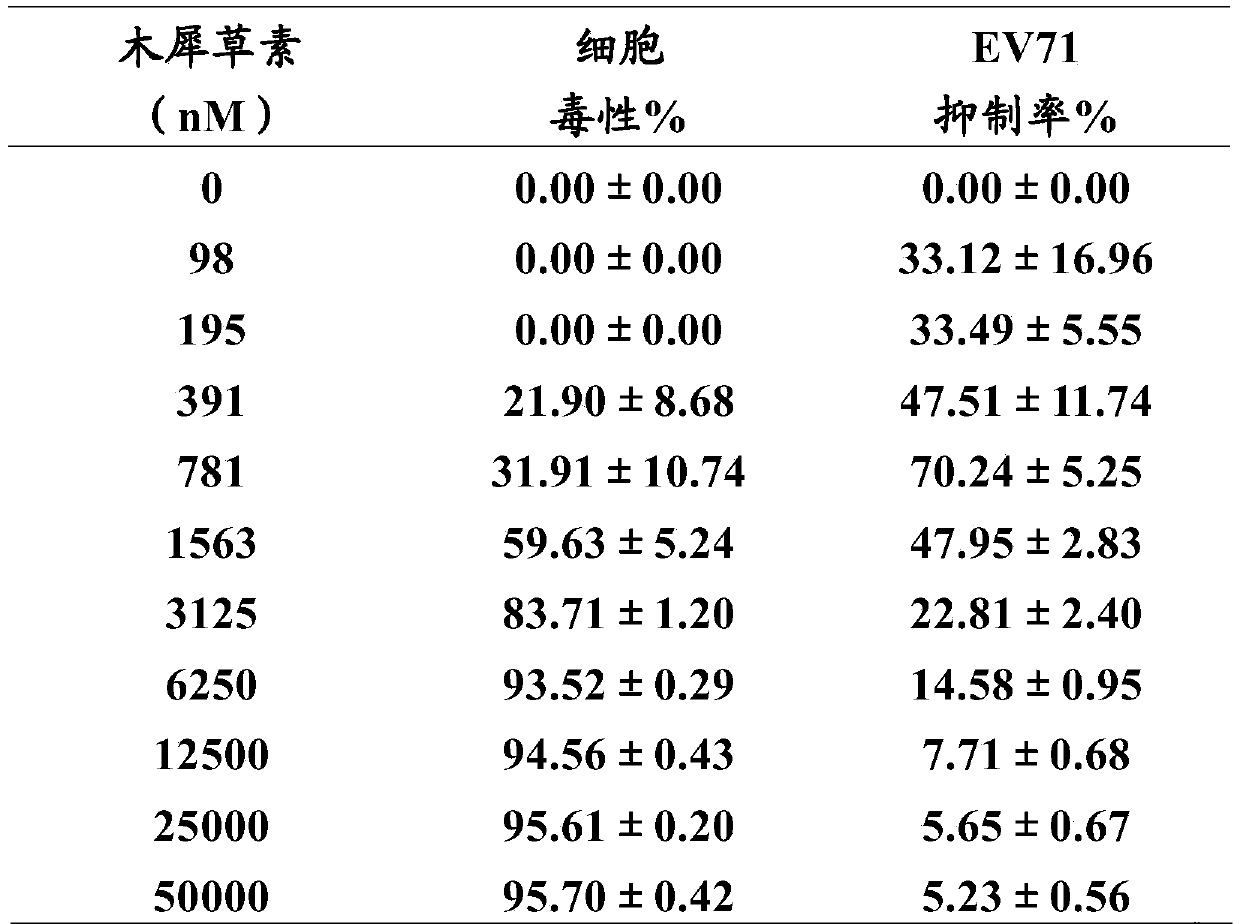
[0036] Example 2: Cytotoxicity of Cinobufagin and Esterbufagenin on Cells and Inhibition of Cytopathic Effects Caused by Enterovirus 71
[0037] Take RD cells and RDS cells in the logarithmic growth phase, and divide them into 3×10 4 pcs / hole and 6×10 4 The concentration of cells / well was inserted into a 96-well culture plate and cultured for 24 hours. Both RD cells and RDS cells were incubated at 37 °C and 5% CO 2 Under culture, wherein the culture medium used for cultivating RD cells is DMEM medium supplemented with 10% fetal bovine serum (referred to as 10% FBS-DMEM, FBS (fetal bovine serum) and DMEM are both purchased from Sigma), cultivating RDS cells The culture medium used was 10% FBS-DMEM containing 0.5 μg / ml puromycin (purchased from Clontech).
[0038] Add 50 μL of DMEM cell culture medium (purchased from Sigma) containing different concentrations of cinobufagin or esterbufagenin (purchased from China Institute for the Control of Pharmaceutical and Biological Prod...
Embodiment 3
[0060] Example 3: Inhibition of Cinobufagin and Esterbufagenin on Plaque Formation Caused by EV71
[0061] Take the RDS cells in the logarithmic growth phase, and use 6×10 5 The concentration of each well was inserted into a 12-well culture plate and cultured for 24 hours.
[0062] Aspirate the medium from each well, add 300 μL of DMEM cell culture medium containing different concentrations of cinobufacien to make the final concentration 62.50nM, 31.25nM, 15.63nM, 7.81nM, 3.90nM or add 300μL of cinobufacin containing different concentrations Base DMEM cell culture solution, so that the final concentration is 1563nM, 781nM, 391nM, 195nM, 98nM, each concentration is set up 3 duplicate wells; add 300μ LEV71 (100PFU / hole) virus infection to each hole at the same time of adding medicine; And set only Add the cell control of DMEM cell culture medium and the virus control of only adding EV71 infection; after continuing to culture the cells for 1 hour, aspirate the supernatant of th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com