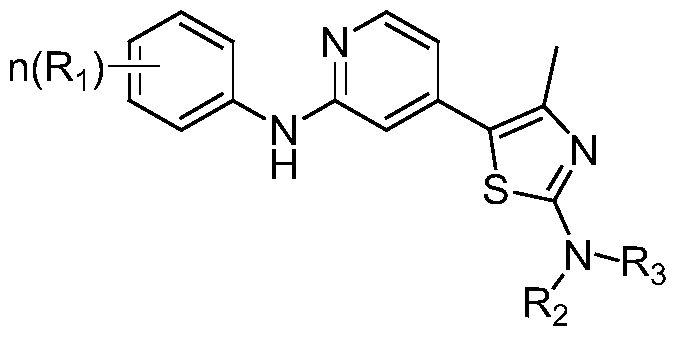
Containing 2-anilino-4-thiazolyl pyridine derivative and its preparation method, pharmaceutical composition and application
A technology of methylthiazole and pyridine, applied in the field of treating tumor diseases
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
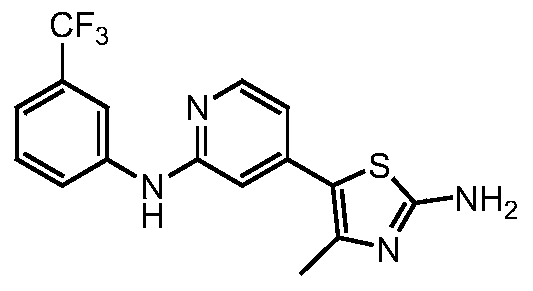
[0214] Example 1.2-(m-trifluoromethylanilino)-4-(2-amino-4-methylthiazol-5-yl)pyridine
[0215]
[0216] 2-Bromo-4-(2-amino-4-methylthiazol-5-yl)pyridine (270mg, 1mmol) was added to 10ml of mixed solvent (1N HCl / H 2 O / Dioxane=2 / 4 / 1), heated to 80°C with stirring, added m-trifluoromethylaniline (242mg, 1.5mmol) after dissolution, raised the temperature, and refluxed until the reaction was complete. Cool to room temperature, pour the reaction solution into saturated aqueous sodium bicarbonate solution, filter, collect the solid, and purify by column chromatography to obtain the product 2-(m-trifluoromethylanilino)-4-(2-amino-4-methyl thiazol-5-yl)pyridine. 1 H-NMR(400MHz,DMSO-d6):δ(ppm):9.43(s,1H,-N H -),9.23(s,1H,Ar H ),8.14(d,1H,Ar H ),7.86(d,1H,Ar H ),7.47(t,1H,Ar H ),7.26(s,2H,-N H 2 ),7.18(d,1H,Ar H ),6.81(s,1H,Ar H ),6.77(d,1H,Ar H ),2.32(s,3H,-C H 3 ); m / z351.0[M+H] + .
Embodiment 2
[0217] Example 2.2-(2-chloro-6-methylanilino)-4-(2-amino-4-methylthiazol-5-yl)pyridine
[0218]
[0219] Replace m-trifluoromethylaniline with 2-chloro-6-methylaniline, and refer to the operation process of Example 1 to obtain 2-(2-chloro-6-methylanilino)-4-(2-amino-4 -Methylthiazol-5-yl)pyridine. 1 H-NMR(300MHz,DMSO-d6):δ(ppm):8.30(s,1H,-N H -),7.89(d,1H,Ar H ),7.36-7.34(d,1H,Ar H ),7.26-7.24(d,1H,Ar H ),7.19-7.14(m,3H,Ar H ,-N H 2 ),6.60-6.58(dd,1H,Ar H ),6.37(s,1H,Ar H ),2.23(s,3H,-C H 3 ),2.18(s,3H,-C H 3 ); m / z331.0[M+H] + .
Embodiment 3
[0220] Example 3.2-(2-fluoroanilino)-4-(2-amino-4-methylthiazol-5-yl)pyridine
[0221]
[0222] Replace m-trifluoromethylaniline with 2-fluoroaniline, and refer to the operation process of Example 1 to obtain 2-(2-fluoroanilino)-4-(2-amino-4-methylthiazol-5-yl)pyridine . 1 H-NMR(300MHz,DMSO-d6):δ(ppm):8.71(s,1H,-N H -),8.26-8.20(m,1H,Ar H ),8.05(d,1H,Ar H ),7.22-7.09(m,4H,-N H 2 , Ar H ),6.99-6.93(m,2H,Ar H ),6.73-6.70(m,1H,Ar H ),2.30(s,3H,-C H 3 ); m / z301.0[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com