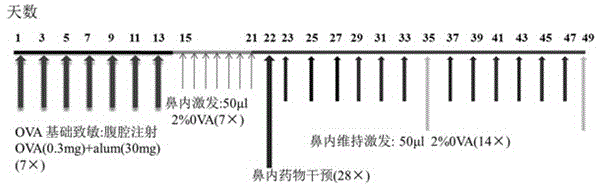
Applications of 18 beta-glycyrrhetinic acid in preparing drugs used for treating allergic rhinitis
A technology for allergic rhinitis and glycyrrhetinic acid, which is applied in the field of medical applications and can solve problems such as undiscovered
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1、18
[0050] The preparation of embodiment 1,18β-sodium glycyrrhetinate nasal drops
[0051] Weigh 8mg of 18β-sodium glycyrrhetinate, dissolve it in 0.267mL of normal saline, and magnetically stir to help dissolve; then use 0.033mL of water for injection to adjust the osmotic pressure to 280~310mmol / L, and adjust the pH to about 7.5 with an appropriate amount of hydrochloric acid, so that the 18β-licorice The final mass concentration of sodium hypochlorite was 2.67%. Inject 10ml of nasal drops into a special plastic bottle, seal and pack, and obtain 2.67% 18β-sodium glycyrrhetinate nasal drops for allergic rhinitis with a specification of 10ml / piece.
[0052] Method of use: nasal drops, 3 times / day, a course of treatment every 2 weeks, generally 1~2 courses of treatment.
Embodiment 2
[0053] Embodiment 2, the preparation of allergic rhinitis drops
[0054] Weigh 4mg of 18β-sodium glycyrrhetinate, dissolve it in 0.267mL of normal saline, and magnetically stir to aid dissolution; use 0.033ml of water for injection to adjust the osmotic pressure to 280~310mmol / L, and adjust the pH to about pH7.5 with an appropriate amount of hydrochloric acid, so that 18β- The final mass concentration of sodium glycyrrhetinate is about 1.34%. Inject 10ml of nasal drops into a special plastic bottle, seal and pack, and obtain 1.24% 18β-sodium glycyrrhetinate nasal drops for allergic rhinitis with a specification of 10ml / piece.
[0055] Method of use: nasal drops, 3 times / day, a course of treatment every 2 weeks, generally 1~2 courses of treatment.
Embodiment 3
[0056] Embodiment 3, the preparation of allergic rhinitis spray
[0057] Weigh 8mg of 18β-sodium glycyrrhetinate, dissolve it in 0.267mL of normal saline, and magnetically stir to help dissolve; then use 0.033mL of water for injection to adjust the osmotic pressure to 280~310mmol / L, and adjust the pH to about 7.5 with an appropriate amount of hydrochloric acid, so that the 18β-licorice The final mass concentration of sodium hypochlorite was 2.67%. Inject 10ml of micro-scaled special plastic bottle nasal spray, seal and pack, and get 2.67% 18β-sodium glycyrrhetinate allergic rhinitis treatment nasal spray with a specification of 10ml / piece.
[0058] Nasal spray nasal spray, 3 times / day, a course of treatment every 2 weeks, generally 1~2 courses of treatment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com