Combined evaluation model for bioavailability and toxicity of arsenic in foods on human body
A bioavailability and evaluation model technology, applied in the field of joint evaluation model, can solve the problems of lack of liver environment, differences in arsenic absorption process, differences in actual bioavailability of arsenic, etc., and achieve the effect of sensitive and efficient evaluation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
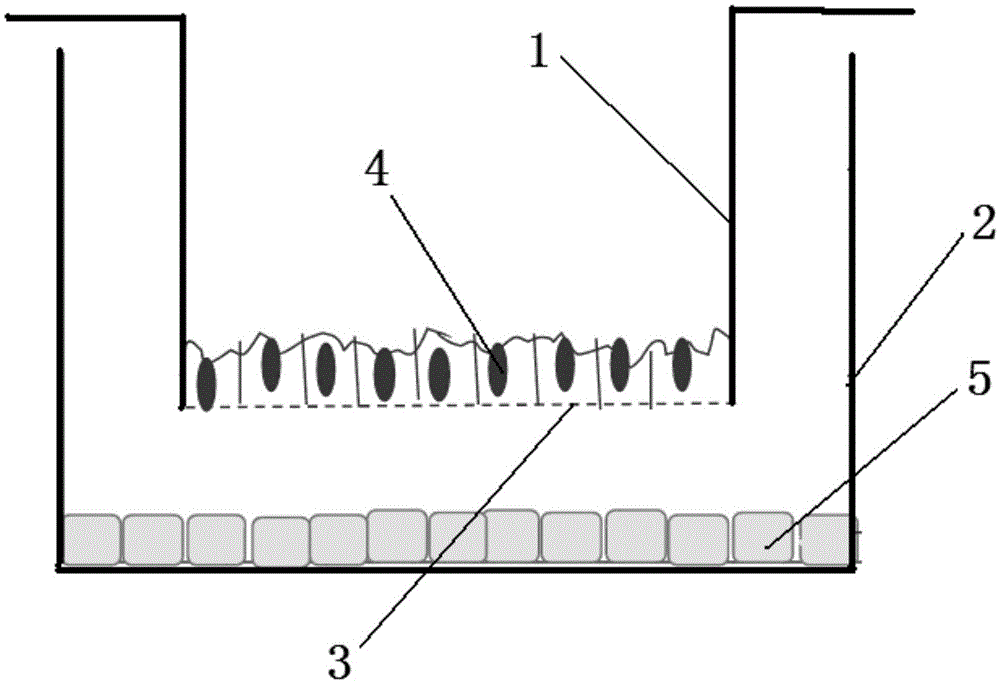
Image
Examples
Embodiment 1
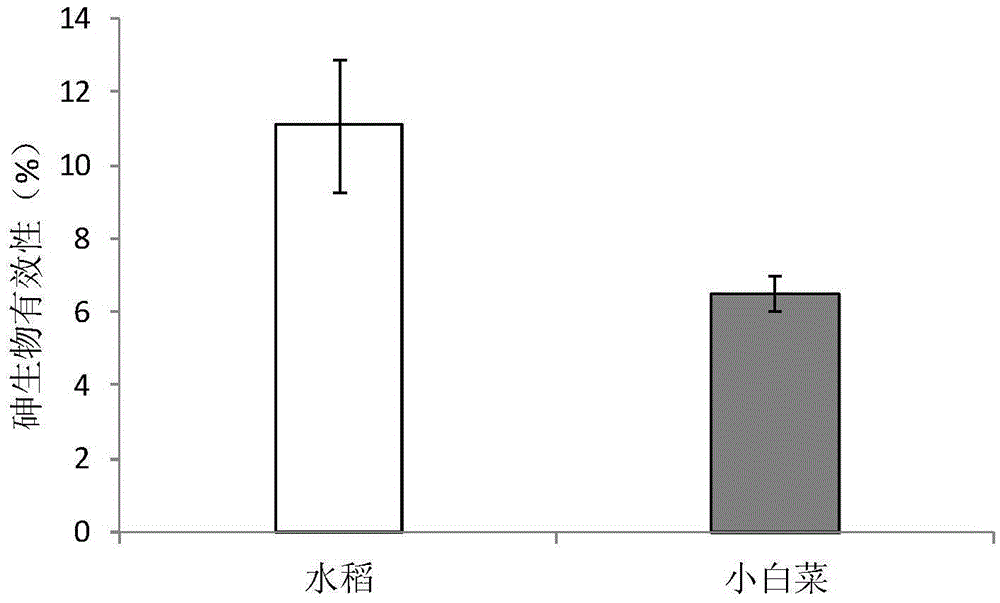
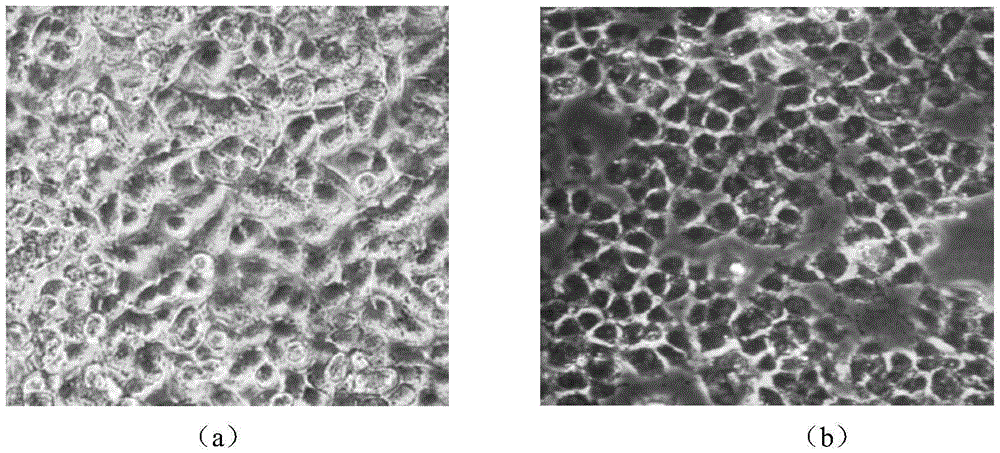
[0046] Examples of the bioavailability and toxicity of arsenic in rice and vegetables measured by the present invention to the human body
[0047] 1. Test material
[0048] 1.1 Cell lines were purchased from the Shanghai Cell Bank of the Chinese Academy of Sciences.
[0049] 1.2 Main reagents and medicines
[0050] DMEM medium, fetal bovine serum, and HBSS were purchased from Gibco,
[0051] Trypsin, pepsin, bile, non-essential amino acids were purchased from Sigma Company,
[0052] Penicillin and streptomycin were purchased from Amresco,
[0053]The rest of the reagents were of domestic analytical grade.
[0054] The buffer used is HBSS solution, pH 7.2-7.35.
[0055] 1.4 Main Equipment and Instruments
[0056] Six-well culture plate (Costar), Transwell insert cell culture dish (polyester membrane, pore size 0.4 μm), ICP-MS (PENexION300X, USA), Millicell-ERS transmembrane resistance meter, ultrapure water system (Millipore, USA) , CO 2 Incubator (Sanyo, Japan), Invert...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com