Sustained release cubic liquid crystal liquid hard capsule and preparation method thereof
A liquid hard capsule and cube technology, which is applied in the field of sustained release, can solve the problems of difficulty in industrialized production of cubic liquid crystal oral preparations, corrosion of the soft capsule wall, and decreased stability of preparations, etc. Drug stability, storage and packaging benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] A slow-release cubic liquid crystal liquid hard capsule, consisting of a capsule shell and a capsule content, the capsule content is prepared from the following raw materials:
[0057]
[0058] The preparation of the sustained-release cubic liquid crystal liquid hard capsules of three prescriptions of the present embodiment comprises the following steps:
[0059] 1) Take the prescribed amount of GMO, heat and melt in a water bath at 40-45°C to obtain liquid GMO;
[0060] 2) Add the prescribed amount of drug powder meloxicam and corresponding auxiliary materials to the liquid GMO obtained in step 1), heat and stir in a water bath at 40-45°C for 1-6 hours, so that the system is in a uniform liquid state, and the contents of the liquid capsule are obtained;
[0061] 3) Fill the liquid capsule content obtained in step 2) into the upright hard capsule shell while it is hot, add a cap to seal it, and stand it upright overnight at room temperature to obtain the slow-release...
Embodiment 2
[0065] A slow-release cubic liquid crystal liquid hard capsule, consisting of a capsule shell and a capsule content, the capsule content is prepared from the following raw materials:
[0066]
[0067] The preparation method of the sustained-release cubic liquid crystal liquid hard capsules of the four prescriptions of this embodiment is the same as that of Embodiment 1.
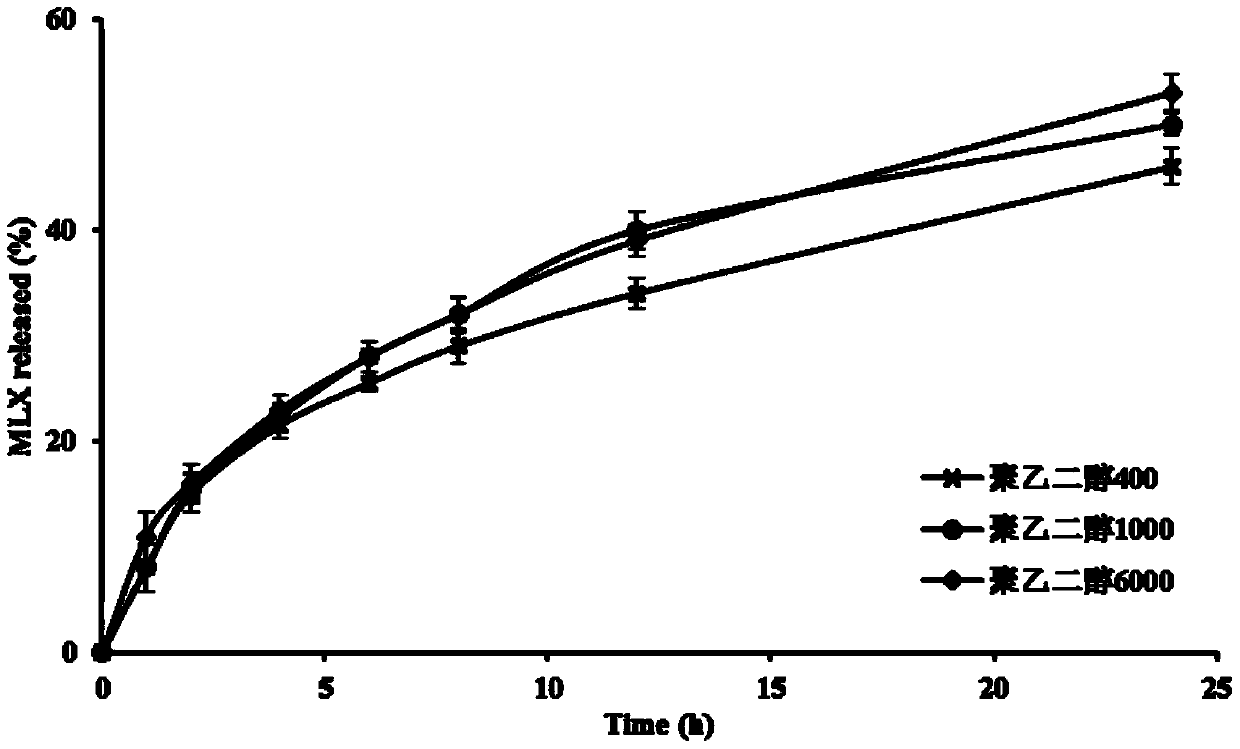
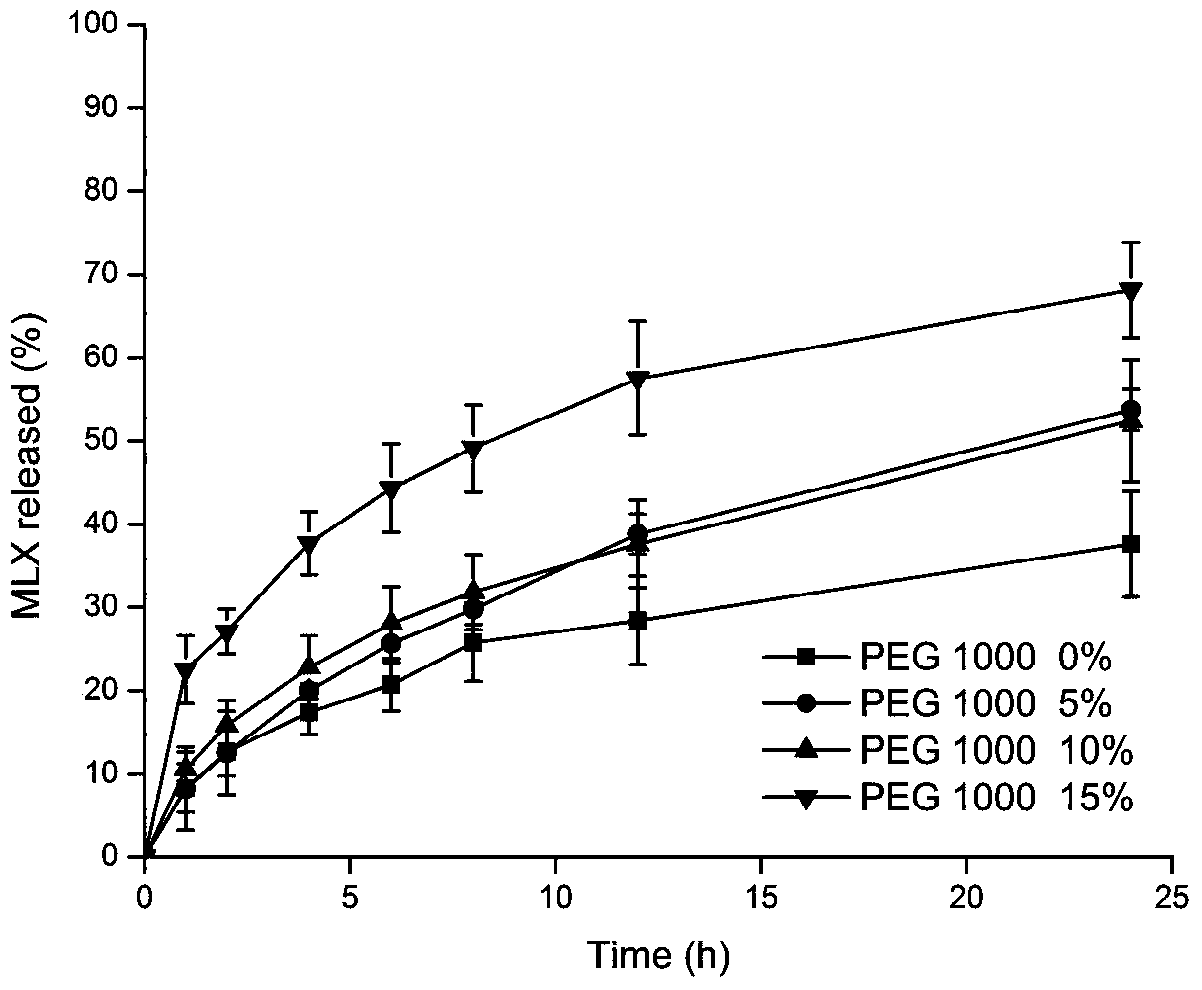
[0068] The slow-release cubic liquid crystal liquid hard capsule prepared in this implementation is carried out release degree measurement, and assay method is the same as embodiment 1, and test result is as follows: figure 2 shown.
[0069] The inventor has found through a large number of experimental studies that the content of polyethylene glycol 1000 has a great influence on the release of the sustained-release cubic liquid crystal liquid hard capsule, and if the content is too high, the drug in the sustained-release cubic liquid crystal liquid hard capsule will appear to a certain extent. Burst rele...
Embodiment 3
[0071] A slow-release cubic liquid crystal liquid hard capsule, consisting of a capsule shell and a capsule content, the capsule content is prepared from the following raw materials:
[0072]
[0073] The preparation method of the sustained-release cubic liquid crystal liquid hard capsules of the four prescriptions of this embodiment is the same as that of Embodiment 1.
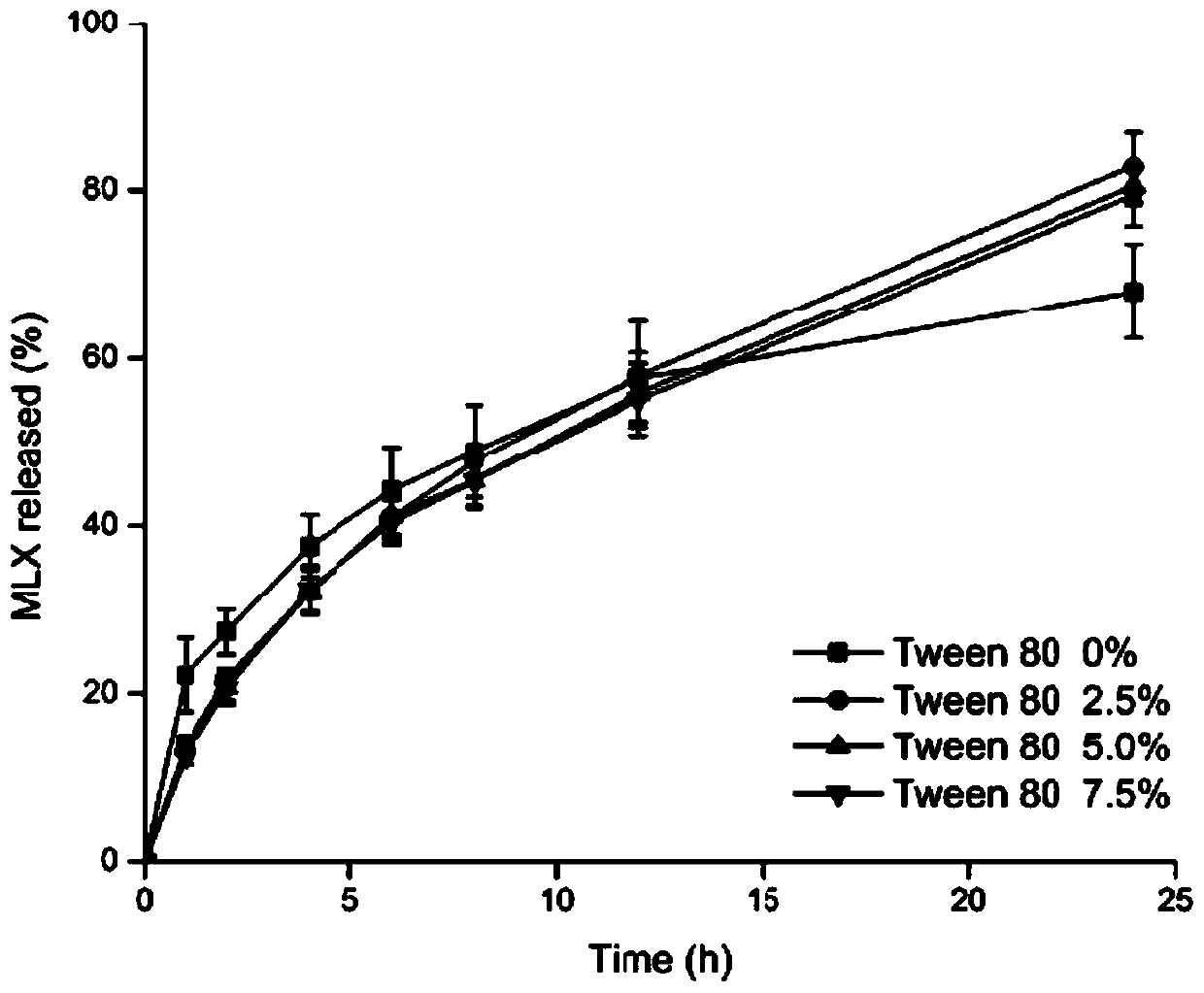
[0074] The slow-release cubic liquid crystal liquid hard capsules prepared by the present embodiment are tested for release, and the assay method is the same as in Example 1, and the test results are as follows: image 3 shown.
[0075] Depend on image 3 As a result, it can be seen that the slow-release cubic liquid crystal liquid hard capsules of the present embodiment all have a good sustained-release effect, and the addition of Tween 80 can further significantly improve the drug release degree of meloxicam sustained-release cubic liquid crystal liquid hard capsules, and further reduce the sudden relea...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com