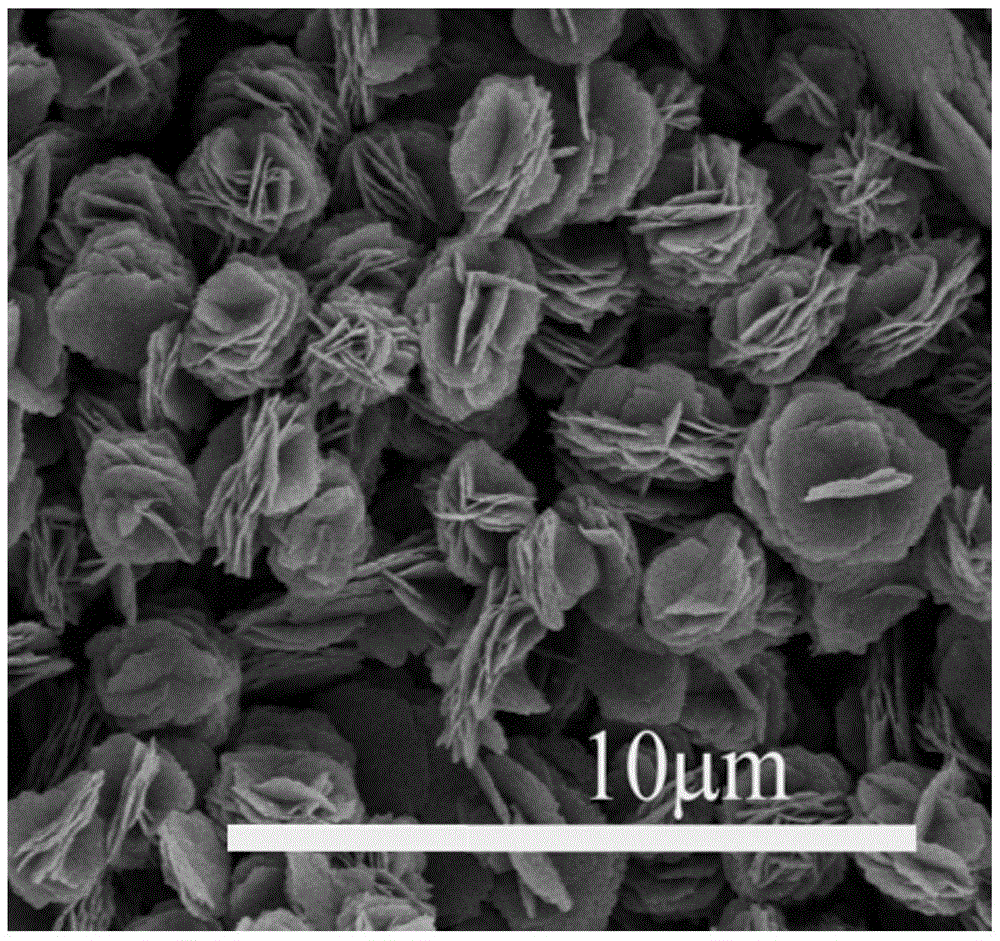
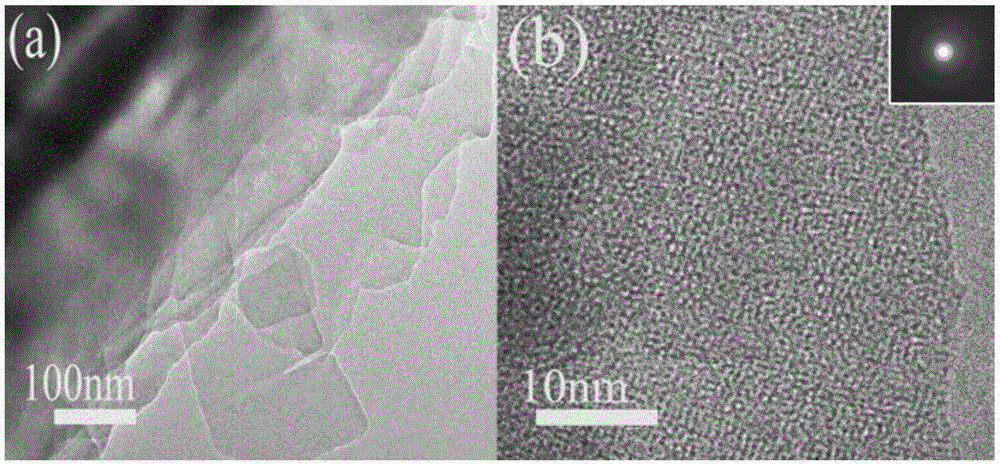
Fullerene derivative microflower and preparation method thereof
A technology of fullerene derivatives and micro-flowers, which is applied in the direction of organic chemistry, etc., can solve the problems of low compound content, the size of micro-flower morphological products cannot adapt to the application, and the effect is affected.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
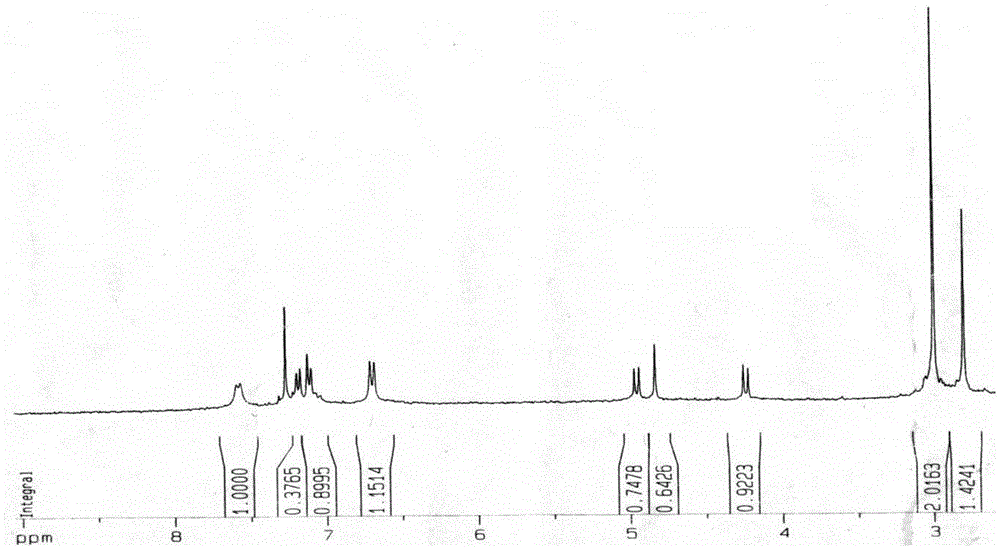
[0028] The preparation of embodiment 1N-methyl-2-[4-dimethylamino] phenyl-3,4-fullerenyl pyrrolidine
[0029] The preparation method of N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine described in this example is prepared according to known methods in the prior art, specifically: Under the protection of argon, the fullerene C was first mixed with a molar ratio of 1:7 60 Dissolve in freshly distilled toluene and stir for 1-2h to give fullerene C 60 toluene solution, and then to the fullerene C 60 Add 4-dimethylaminobenzaldehyde and sarcosine to the toluene solution, and place it under reflux at 120°C for 2-3 hours to obtain a reaction solution, wherein the fullerene C in the reaction solution 60 The molar ratio of , 4-dimethylaminobenzaldehyde and sarcosine is 1:5:3, and then, argon gas is introduced into the reaction solution, and after cooling to room temperature, it is filtered, concentrated and columnar. Chromatographic separation to obtain the reaction conc...
Embodiment 2
[0031] The preparation method of N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine micron flower described in this example comprises the following steps:
[0032] (1) 0.5 g of N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine prepared in Example 1 was mixed with 1L of carbon tetrachloride solvent, Prepare a solution of N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine carbon tetrachloride with a concentration of 0.5g / L for subsequent use;
[0033](2) Get cetyltrimethylammonium bromide and isopropanol solvent and mix, and the obtained concentration is the cetyltrimethylammonium bromide isopropanol solution of 10mmol / L, for subsequent use;
[0034] (3) Get the N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine carbon tetrachloride prepared in step (1) according to the volume ratio of 1:1 solution, mixed with the cetyltrimethylammonium bromide isopropanol solution prepared in step (2) and stirred, the temperature of the reaction system ...
Embodiment 3
[0037] The preparation method of N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine micron flower described in this example comprises the following steps:
[0038] (1) Take N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenyl pyrrolidine 3g prepared in Example 1 and mix with 1L carbon tetrachloride solvent to prepare The N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine carbon tetrachloride solution with a concentration of 3.0 g / L was prepared for subsequent use;
[0039] (2) Get cetyltrimethylammonium bromide and isopropanol solvent and mix, and the obtained concentration is the cetyltrimethylammonium bromide isopropanol solution of 2mmol / L, for subsequent use;
[0040] (3) Get the N-methyl-2-[4-dimethylamino]phenyl-3,4-fullerenylpyrrolidine carbon tetrachloride prepared in step (1) according to the volume ratio of 1:10 solution, mixed with the cetyltrimethylammonium bromide isopropanol solution prepared in step (2) and stirred, the temperature of the react...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com