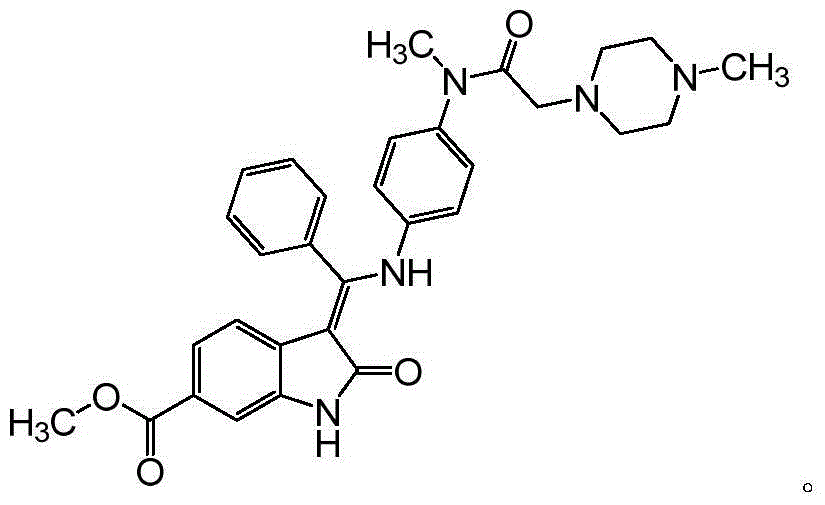
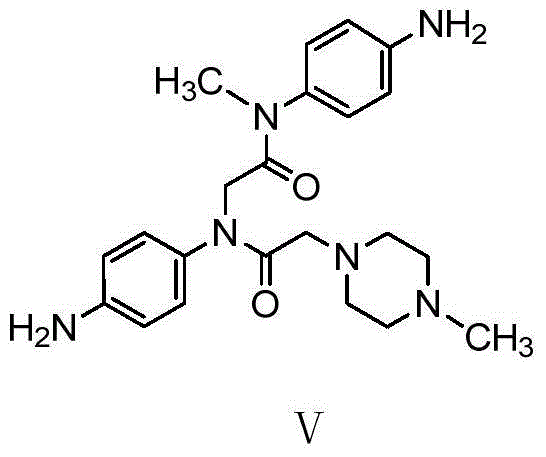
Nintedanib impurity and preparation method and application thereof
A technology of nintedanib, impurity reference substance, applied in the direction of organic chemistry, etc., can solve the problem that impurities are not reported in literature and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
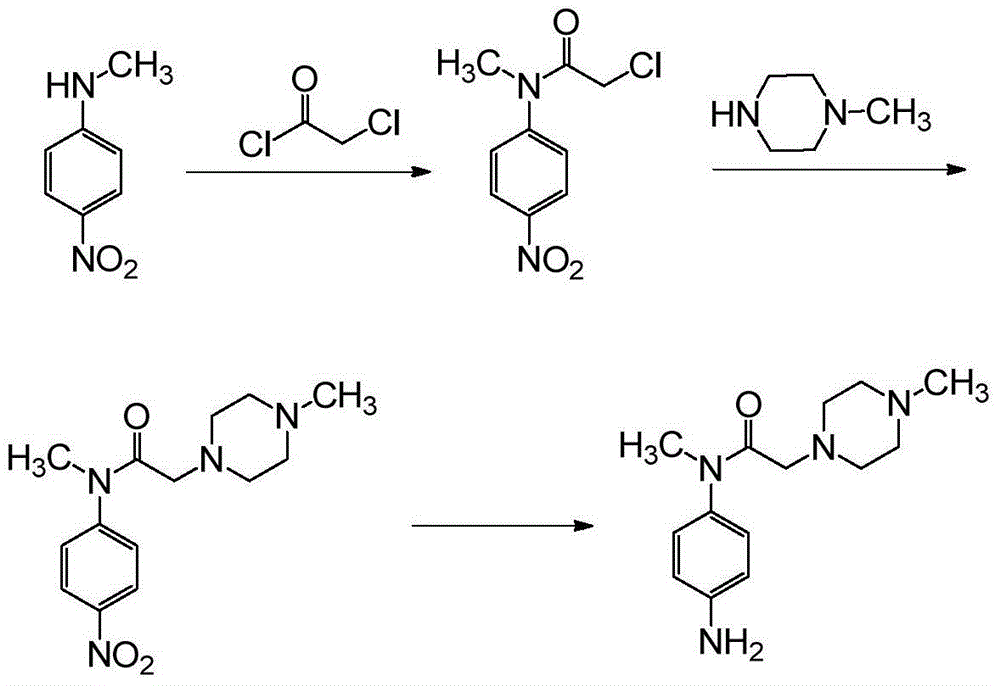
Embodiment 1
[0035] Preparation of 2-chloro-N-methyl-N-(4-nitrophenyl)acetamide
[0036]
[0037] N-methyl-4-nitroaniline (10g, 0.066mol) and ethyl acetate (40mL) were mixed and stirred, heated to 60°C, chloroacetyl chloride (9.2g, 0.081mol) was added dropwise, and refluxed for 5 hours. Cool down, add 30 mL of n-heptane dropwise, filter, and dry the solid in vacuum. Yield 11.5g (yield 76.6%), HPLC purity 98.6%, MS (m / z): 229 ((M+H) + .
Embodiment 2
[0039] Preparation of N-methyl-N-(4-nitrophenyl)-2-(4-nitrophenylamine)acetamide
[0040]
[0041] 2-Chloro-N-methyl-N-(4-nitrophenyl)acetamide (6.9g, 0.0302mol), 4-nitroaniline (4.3g, 0.0311mol), potassium iodide (5.1g, 0.0307mol ), N,N-diisopropylethylamine (3.9g, 0.0302mol) and toluene (50mL) were mixed and stirred, heated at 100°C for 3 hours, cooled, added 50ml of water, stirred, filtered, and dried in vacuum. Yield 6.8g, (yield 68.2%), HPLC purity 99.2%, MS (m / z): 331 (M+H) + .
Embodiment 3
[0043] Preparation of 2-chloro-N-(2-(methyl(4-nitrophenyl)amine)-2-oxoethyl)-N-(4-nitrophenyl)acetamide
[0044]
[0045] N-methyl-N-(4-nitrophenyl)-2-(4-nitrophenylamine)acetamide (6g, 0.018mol), ethyl acetate (60mL) and chloroacetyl chloride (2.37g , 0.021mol) were mixed and stirred, heated to 60°C, and reacted for 6 hours. Cool down, filter, and dry the solid in vacuo. Yield 3.9g (yield 53.3%), HPLC purity 98.7%, MS (m / z): 407 (M+H) + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com