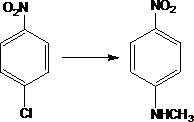
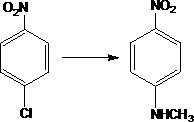
Preparation method of N-methyl-4-nitroaniline
A technology of p-nitroaniline and p-nitrochlorobenzene, which is applied in the field of preparation of N-methyl-p-nitroaniline, can solve the problems of high toxicity of dimethyl sulfate, difficulty in product quality assurance, and high safety risks for operators, etc. problems, to achieve the effect of fewer raw material varieties, stable product quality, and less raw material residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] Add 300g of p-nitrochlorobenzene and 700g of methylamine aqueous solution with a concentration of 40% to a 1L autoclave, add 2.0g of copper sulfate and 0.4g of cobalt sulfate, seal the autoclave, and replace the air in the autoclave with nitrogen gas. After the replacement, the temperature is raised, the temperature is 150°C, the temperature is kept at 1.4MPa for 2.8 hours, the temperature is lowered to 65°C and the pressure is released, and the discharged methylamine is absorbed through the absorption tower. Continue to cool down to normal temperature after depressurization, put the material in the autoclave into a filter and suction filter to obtain 280 g of N-methyl-p-nitroaniline crude product (calculated on dry basis).
[0021] Add 280g of crude N-methyl p-nitroaniline, 1100g of propylene glycol and 2g of sodium bisulfite into a three-necked flask, raise the temperature to 65°C and stir for half an hour, then cool down to 30°C, filter, wash, and dry to obtain N-meth...
Embodiment 2
[0023] Add 150g of p-nitrochlorobenzene and 450g of methylamine aqueous solution with a concentration of 40% to a 1L autoclave, add 1.4g of copper acetate and 0.3g of cobalt acetate, seal the autoclave, and replace the air in the autoclave with nitrogen gas to replace After the temperature is raised, the temperature is 150°C, and the pressure is about 1.4MPa, and the temperature is kept for 2.1hr. After cooling down to 65°C, the pressure is released, and the released free methylamine is absorbed through the absorption tower. Continue to cool down to 30° C., put the material in the autoclave into a filter for suction filtration, and obtain 138 g of crude N-methyl-p-nitroaniline (calculated on dry basis).
[0024] Add the crude product of N-methyl p-nitroaniline, 480g of butanol and 1.5g of sodium hydrosulfide into a three-necked flask, raise the temperature to 65°C and stir for half an hour, cool down to below 30°C, filter, wash, and dry to obtain N-methyl p-nitroaniline 135g o...
Embodiment 3
[0026] Add 200g of p-nitrochlorobenzene and 400g of methylamine aqueous solution with a concentration of 40% to a 1L autoclave, add 1.5g of copper acetate and 0.3 g of cobalt acetate, seal the autoclave, and pass nitrogen into the autoclave to replace the air in the autoclave. After the temperature rises, the temperature is 140°C, and the pressure is about 1.1MPa, and the temperature is kept for 2 hours. After cooling down to 40°C, the pressure is released, and the discharged free methylamine is absorbed through the absorption tower. Continue to cool down to 25° C., put the material in the autoclave into a filter for suction filtration, and obtain 185 g of crude N-methyl-p-nitroaniline (calculated on a dry basis).
[0027] Add the crude product of N-methyl p-nitroaniline, 555g of butanol and 0.18g of sodium hydrosulfide into a three-necked flask, raise the temperature to 60°C and stir for half an hour, then cool down to below 15°C, filter, wash, and dry to obtain N-methyl p-nit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com