A kind of matrine osmotic pump controlled-release tablet and preparation method thereof
An osmotic pump controlled release and matrine technology, which is applied in the directions of osmotic delivery, pharmaceutical formulations, and medical preparations with inactive ingredients, etc. The sudden release phenomenon and other problems can inhibit the activity of collagen, avoid the phenomenon of peak and valley, and reduce the number of times of medication.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
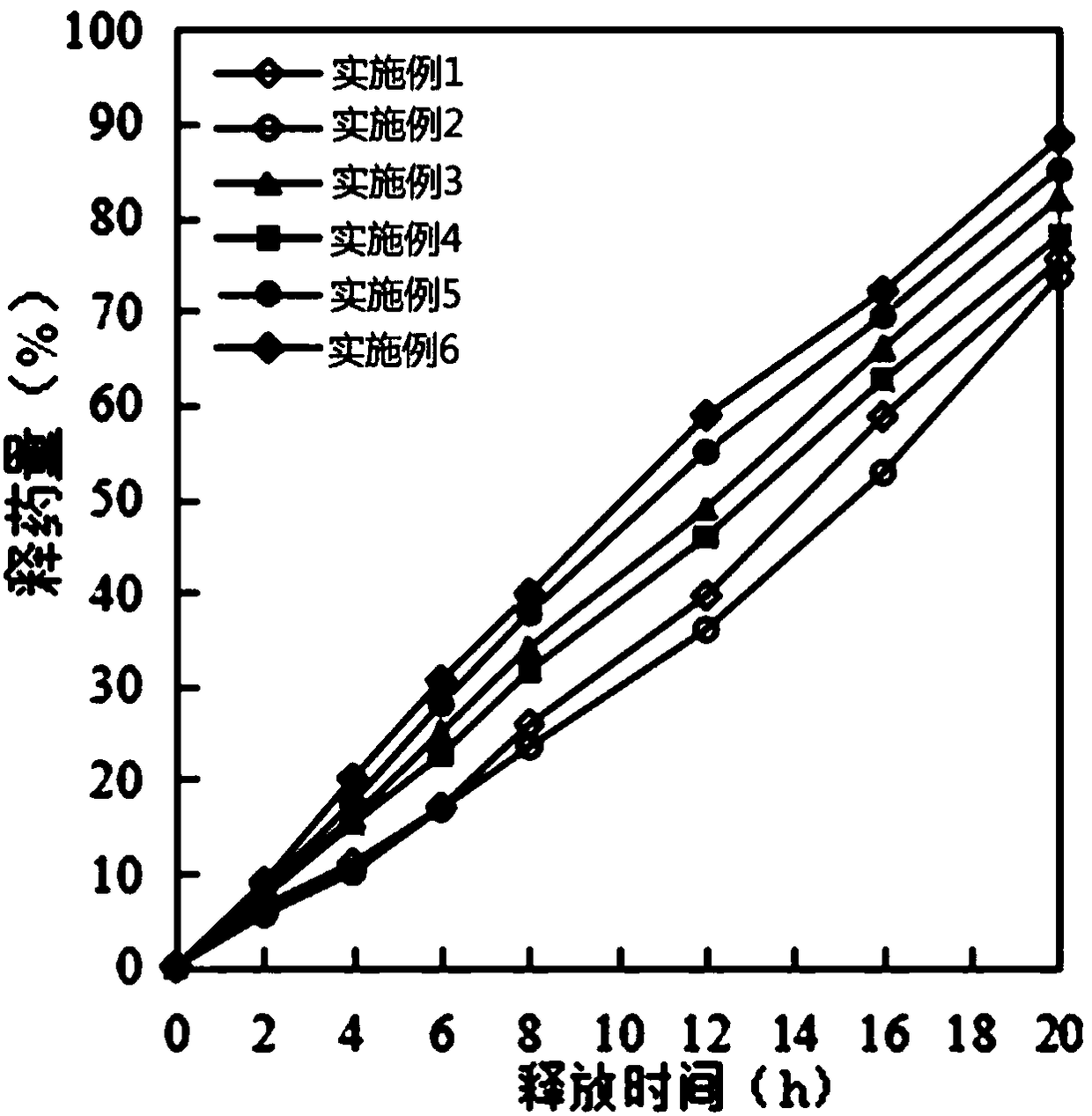
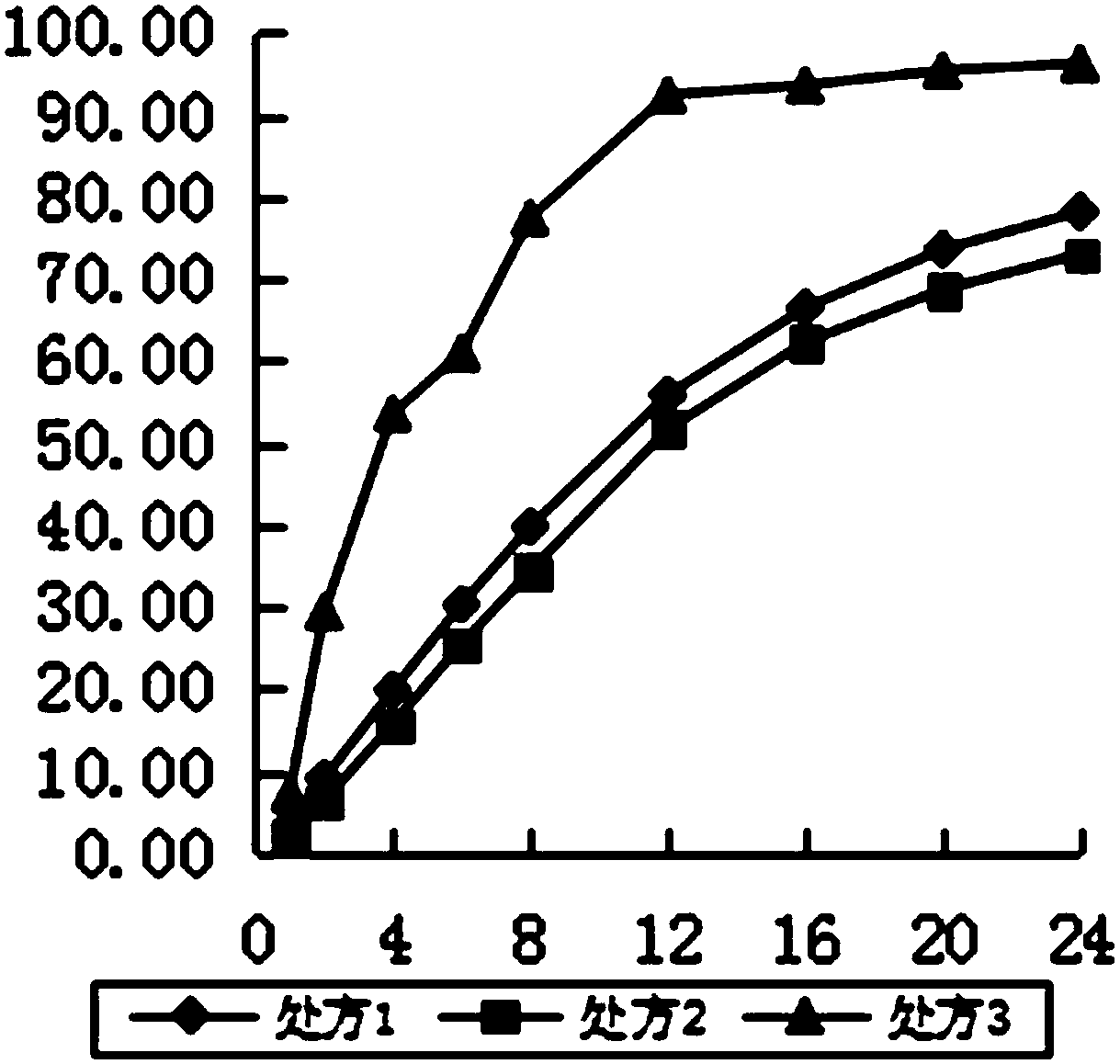
Examples
Embodiment 1
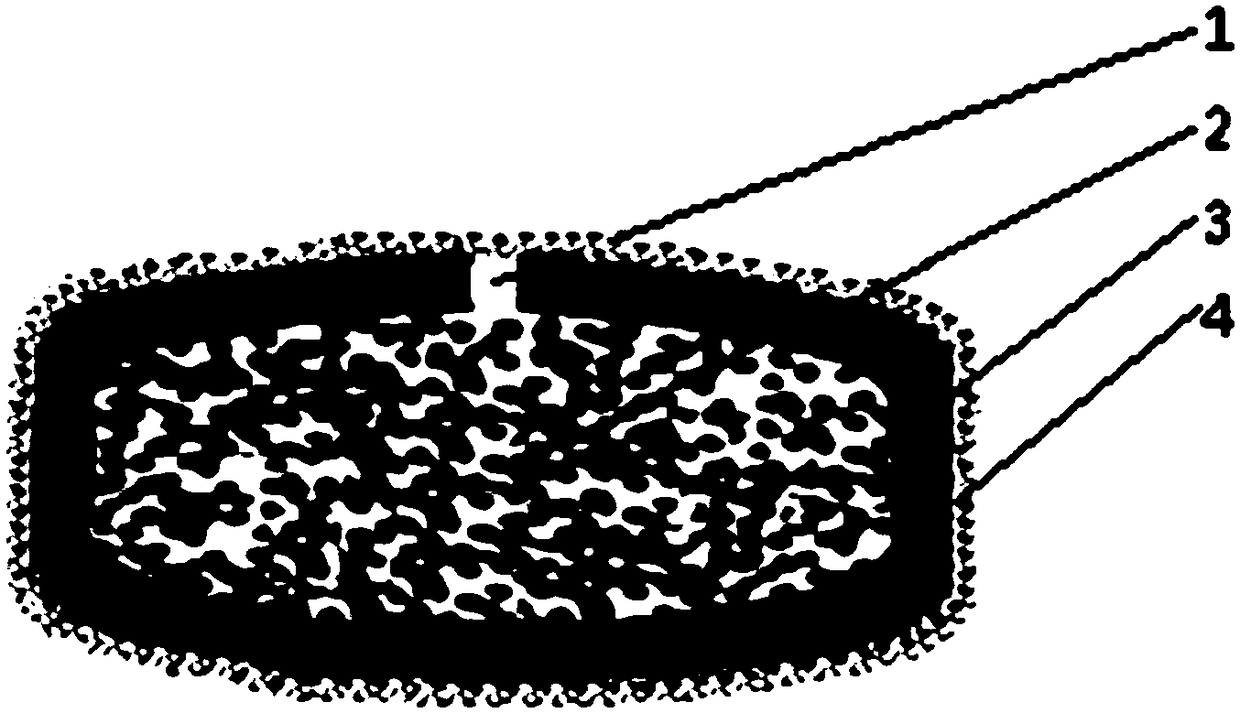
[0066] See figure 1 As shown, a matrine osmotic pump controlled-release tablet is composed of a plain tablet and a moisture-proof protective film 4 wrapped outside the plain tablet. The plain tablet is composed of a tablet core 2 and a semipermeable membrane 3 wrapped outside the tablet core 2. A drug release hole 1 is provided on the semipermeable membrane 3; the drug release hole 1 is formed by laser drilling, and the size of the hole is 0.5mm.
[0067] Tablet core 2 is made up of the raw material of following weight percentage content:
[0068] 40.00% matrine, 21.00% sodium chloride, 30.00% lactose, 5.00% hydroxypropyl methylcellulose, 3.50% polyvinylpyrrolidone, 0.50% magnesium stearate; the weight of the tablet core is 450.00 mg.
[0069] The semipermeable membrane coating liquid that prepares semipermeable membrane 3 is made up of the raw material of following weight percent content:
[0070] Cellulose acetate 3.50%, polyethylene glycol-4000 0.25%, diethyl phthalate 0....
Embodiment 2
[0083] See figure 1 As shown, a matrine osmotic pump controlled-release tablet is composed of a plain tablet and a moisture-proof protective film 4 wrapped outside the plain tablet. The plain tablet is composed of a tablet core 2 and a semipermeable membrane 3 wrapped outside the tablet core 2. A drug release hole 1 is provided on the semipermeable membrane 3; the drug release hole 1 is formed by laser drilling, and the size of the hole is 0.5mm.
[0084] Tablet core 2 is made up of the raw material of following weight percentage content:
[0085] 40.00% matrine, 25.00% sodium chloride, 26.00% lactose, 4.75% hydroxypropyl methylcellulose, 2.75% polyvinylpyrrolidone, 1.50% magnesium stearate; the weight of the tablet core is 450.00mg.
[0086] The semipermeable membrane coating liquid that prepares semipermeable membrane 3 is made up of the raw material of following weight percent content:
[0087] Cellulose acetate 3.50%, polyethylene glycol-4000 0.25%, diethyl phthalate 0.3...
Embodiment 3
[0091] See figure 1 As shown, a matrine osmotic pump controlled-release tablet is composed of a plain tablet and a moisture-proof protective film 4 wrapped outside the plain tablet. The plain tablet is composed of a tablet core 2 and a semipermeable membrane 3 wrapped outside the tablet core 2. A drug release hole 1 is provided on the semipermeable membrane 3; the drug release hole 1 is formed by laser drilling, and the size of the hole is 0.5mm.
[0092] Tablet core 2 is made up of the raw material of following weight percentage content:
[0093] 60.00% matrine, 15.00% sodium chloride, 20.00% lactose, 2.75% hydroxypropyl methylcellulose, 1.25% polyvinylpyrrolidone, 1.00% magnesium stearate; the weight of the tablet core is 350.00mg.
[0094] The semipermeable membrane coating liquid that prepares semipermeable membrane 3 is made up of the raw material of following weight percent content:
[0095] Cellulose acetate 3.80%, polyethylene glycol-4000 0.27%, diethyl phthalate 0.3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com