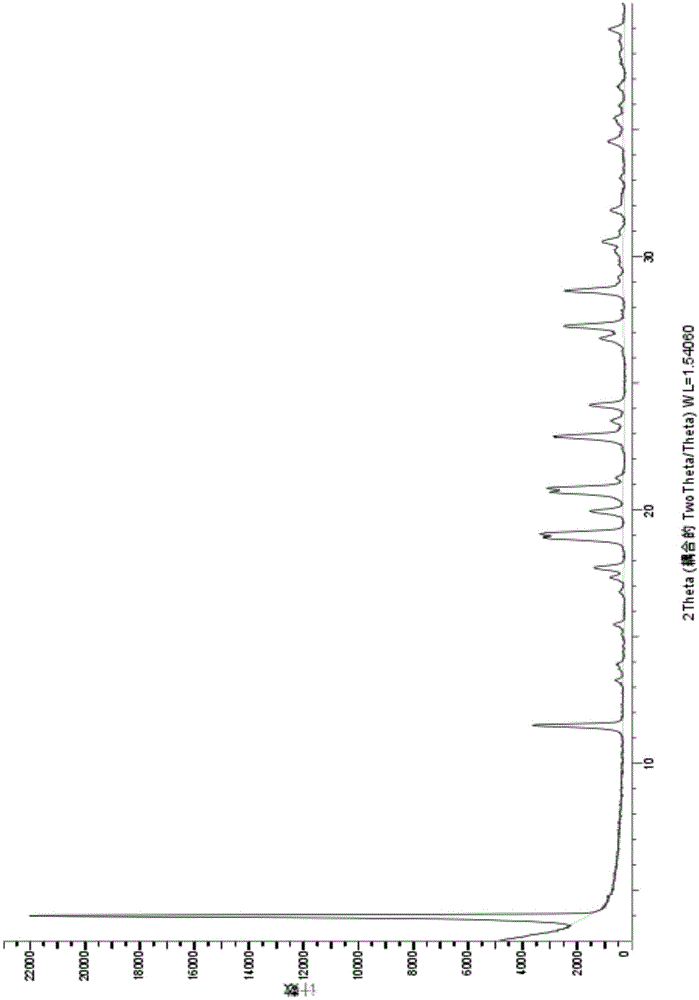
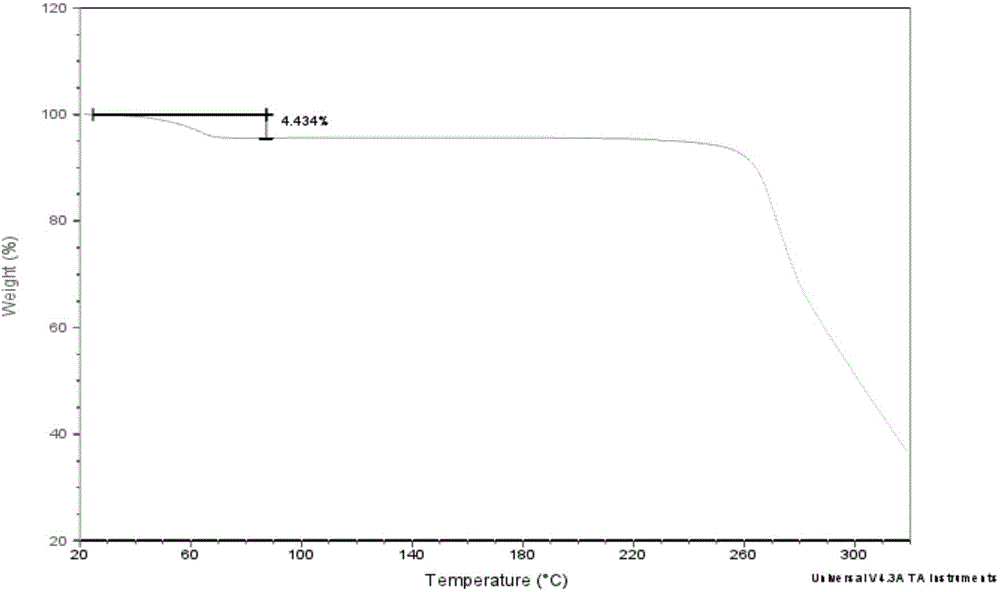
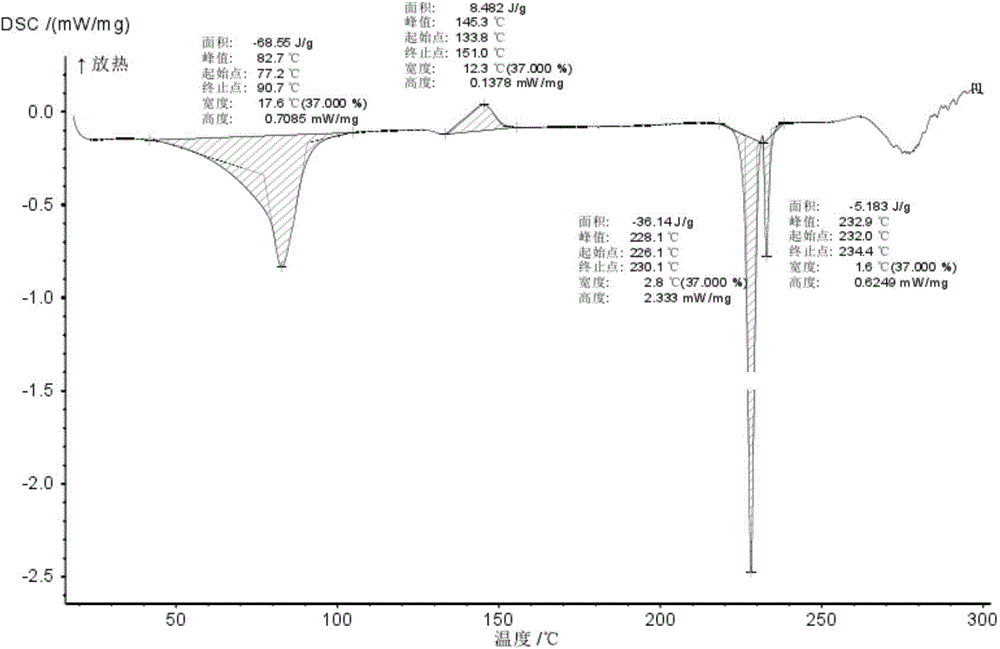
Novel crystal form of Vortioxetine hydrobromate and preparation method for novel crystal form of Vortioxetine hydrobromate
A technology of vortioxetine hydrobromide and cetine hydrobromide compound, which is applied in the field of new crystal form of vortioxetine hydrobromide and its preparation, can solve problems such as inability to achieve simple and stable operation Good performance, to meet the effect of large-scale industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] At room temperature, weigh 1 g of vortioxetine hydrobromide, add it into 6 ml of N,N-dimethylformamide, raise the temperature to 75° C. to dissolve it completely, and filter it. The filtrate was added to 50ml of water cooled to 2°C, a solid was precipitated, and the stirring was continued for 10 minutes, followed by suction filtration, and drying to obtain off-white new crystal form δ of vortioxetine hydrobromide.
Embodiment 2
[0036] At room temperature, weigh 1 g of vortioxetine hydrobromide, add it into 6 ml of N,N-dimethylformamide, raise the temperature to 75° C. to dissolve it completely, and filter it. The filtrate was added to 50ml of water cooled to 5°C, and a solid was precipitated, and the stirring was continued for 20 minutes, followed by suction filtration, and drying to obtain off-white new crystal form δ of vortioxetine hydrobromide.
Embodiment 3
[0038] At room temperature, weigh 100g of vortioxetine hydrobromide, add it into 600ml of N,N-dimethylformamide, raise the temperature to 75°C to completely dissolve it, and filter. The filtrate was added to 5 L of water cooled to 2.5°C, and a solid was precipitated, and the stirring was continued for 2 hours, followed by suction filtration, and drying to obtain off-white vortioxetine hydrobromide new crystal form δ.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com