Anti-TNF-alpha monoclonal antibody chromatographic method
A monoclonal antibody and composite layer technology, which is applied in the field of monoclonal antibody preparation, can solve the problems of increased volume, increased cost, low purity and yield, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] 1) Clarification of cell fluid
[0048] Using an eppendorf centrifuge, centrifuge the CHO cell culture supernatant twice at 10,000 g to remove cells and cell debris, and pass through a 0.2 μm filter membrane to further reduce the turbidity of the sample.
[0049] 2) Composite chromatography
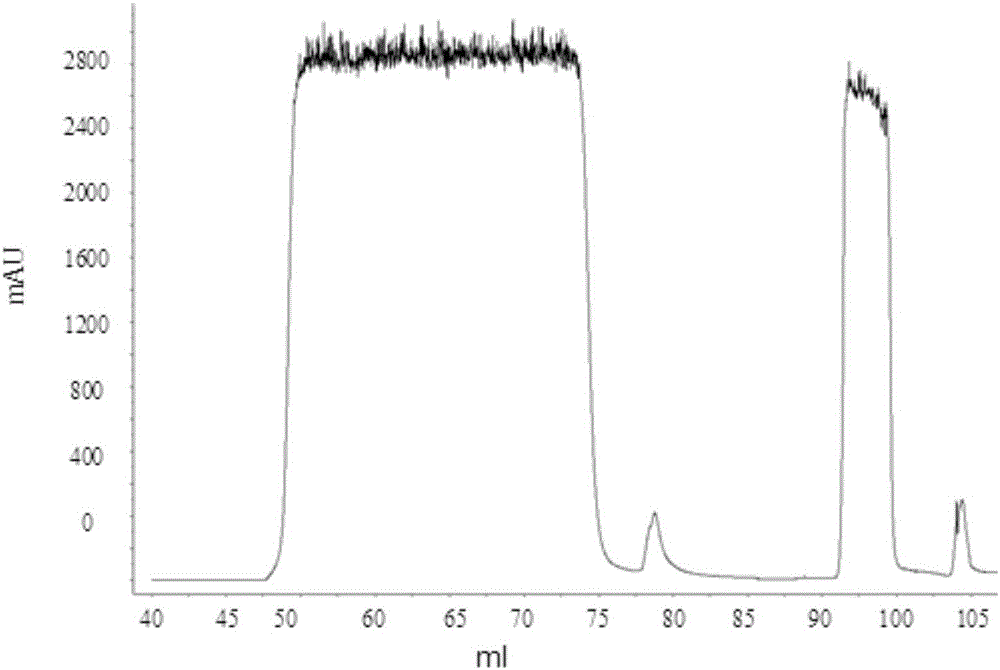
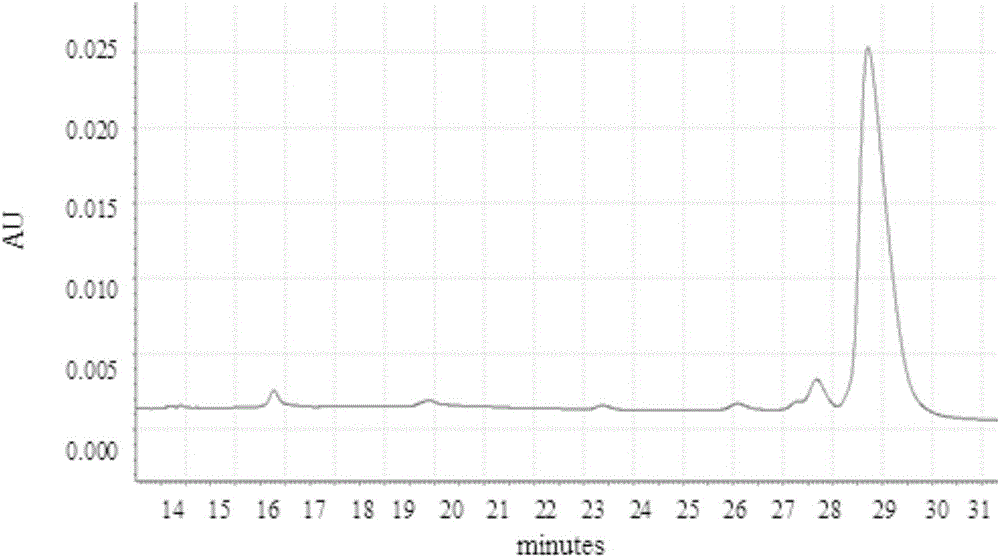
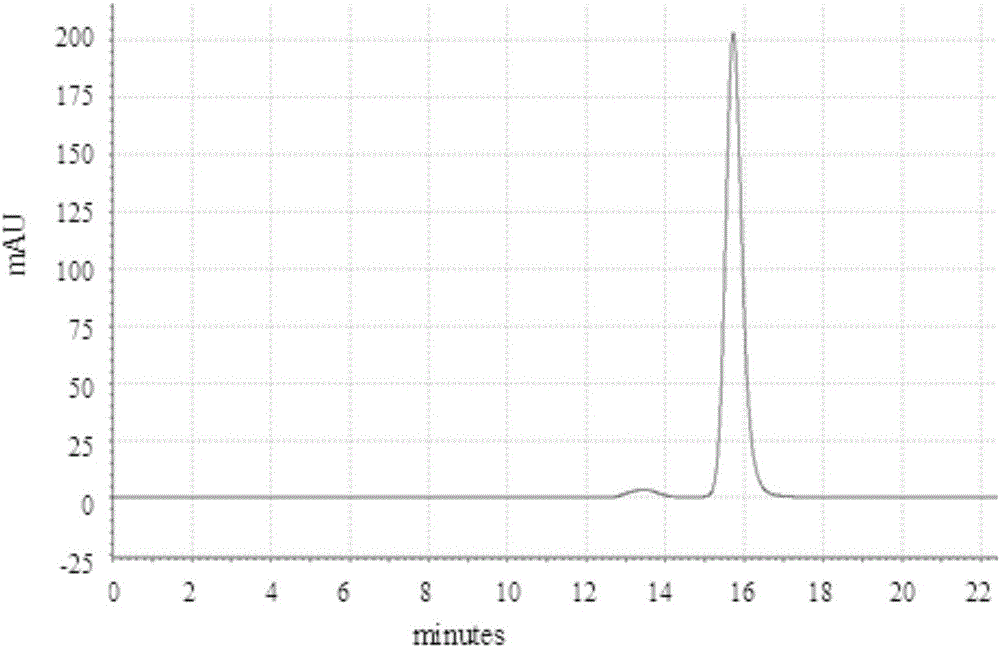
[0050] Using AKTA purifier-100 (GE Healthcare) chromatographic system, 1.2ml MEP (PALL company) composite chromatographic medium was loaded in Tricorn10 / 20 (GE company) chromatographic column. Fully equilibrate the recombination chromatography column with equilibration buffer (50mM PBS pH7.0), and start loading the sample when the UV absorption at 280nm returns to the baseline, and the conductance and pH remain stable, and then wash with the first washing buffer (50mM PBS pH7.0) .0) Wash the unbound whole protein, wait until the UV absorption at 280nm returns to the baseline, and after the conductivity and pH remain stable, then wash with the second washing buffer (50mM PBS pH6.0)...
Embodiment 2
[0072] 1) Clarification of cell fluid
[0073] Using an eppendorf centrifuge, centrifuge the CHO cell culture supernatant twice at 10,000 g to remove cells and cell debris, and pass through a 0.2 μm filter membrane to further reduce the turbidity of the sample.
[0074] 2) Composite chromatography
[0075] Using AKTA purifier-100 (GE Healthcare) chromatographic system, 1.2ml MEP (PALL company) composite chromatographic medium was loaded in Tricorn10 / 20 (GE company) chromatographic column. Fully equilibrate the recombination chromatography column with equilibration buffer (50mM PBS pH7.0), wait for the UV absorption at 280nm to return to the baseline, and start loading the sample when the conductivity and pH remain stable, and then wash with the first washing buffer (50mM PBS pH7.0) to wash the unbound whole protein, wait until the UV absorption at 280nm returns to the baseline, and after the conductivity and pH remain stable, then wash with the second washing buffer (50mM PBS...
Embodiment 3
[0098] 1) Clarification of cell fluid
[0099] Using an eppendorf centrifuge, centrifuge the CHO cell culture supernatant twice at 10,000 g to remove cells and cell debris, and pass through a 0.2 μm filter membrane to further reduce the turbidity of the sample.
[0100] 2) Composite chromatography
[0101] Using AKTA purifier-100 (GE Healthcare) chromatographic system, 1.2ml MEP (PALL company) composite chromatographic medium was loaded in Tricorn10 / 20 (GE company) chromatographic column. Fully equilibrate the recombination chromatography column with equilibration buffer (50mM PBS pH7.0), and start loading the sample when the UV absorption at 280nm returns to the baseline, and the conductance and pH remain stable, and then wash with the first washing buffer (50mM PBS pH7.0) .0) Wash the unbound whole protein, wait until the UV absorption at 280nm returns to the baseline, and after the conductance and pH remain stable, then wash with the second washing buffer (50mM PBS pH6.0),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com