A kind of acid-sensitive amphiphilic stearic acid amidated dextran polymer nanomicelle and preparation method thereof
A technology of stearic acid amidated glucan and fatty acid amidated glucan is applied in the field of drug-loaded micelles and their preparation, which can solve the problems of no pH response of stearic acid glucan micelles and the like, and achieve drug-loaded micelles. Release the effect of enhancing and enhancing solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
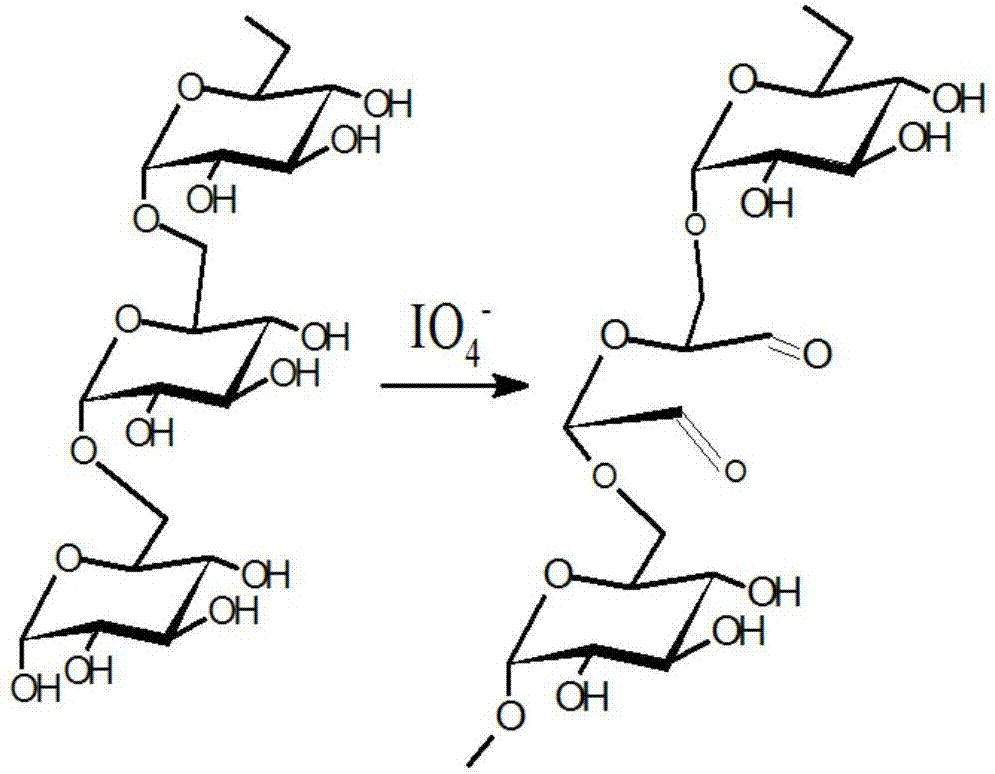
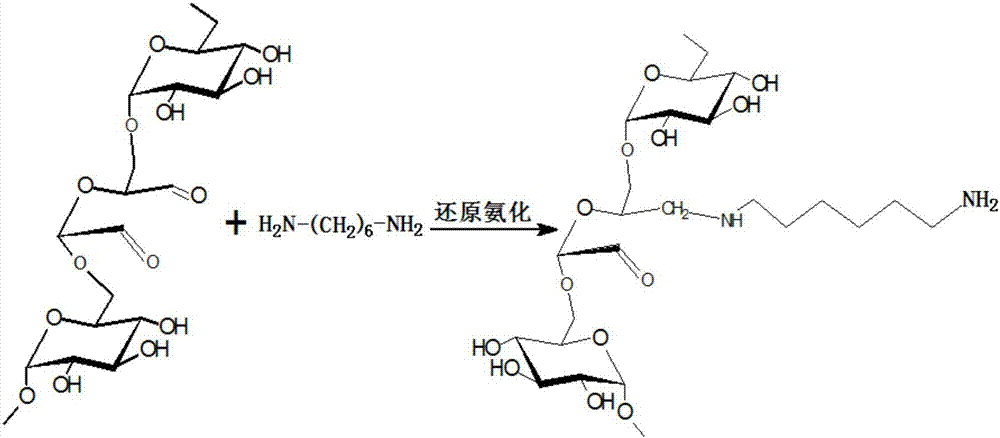
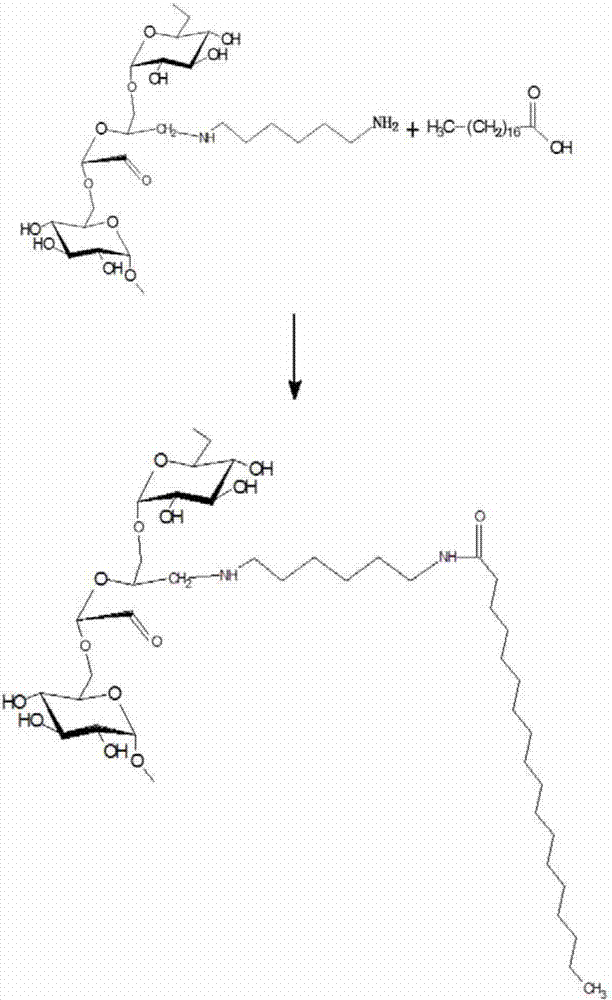
[0036] Specific embodiment 1: This embodiment is an acid-sensitive amphiphilic stearic acid amidated dextran polymer nanomicelle, which uses dextran as a raw material, and introduces active aldehydes on the surface of the dextran structure through an oxidation reaction base to obtain polyaldehyde dextran, and then react polyaldehyde dextran with hexamethylenediamine to generate hexamethylenediamine-polyaldehyde dextran, and the hexamethylenediamine-polyaldehyde dextran has a stable carbon-nitrogen unit bond structure, and carry active amino groups; hexamethylenediamine-polyaldehyde dextran is acylated with stearic acid to obtain stearic acid amidated dextran polymer, and the amphiphile of stearic acid amidated dextran polymer Self-assembled into nano-micelles in water, that is, acid-sensitive amphiphilic stearic acid amidated dextran polymer nano-micelles.
specific Embodiment approach 2
[0037] Specific embodiment two: This embodiment is a preparation method of acid-sensitive amphiphilic stearic acid amidated dextran polymer nanomicelle, which is specifically completed according to the following steps:
[0038] 1. Preparation of polyaldehyde dextran: ①. Dissolve dextran in distilled water to obtain a dextran aqueous solution with a concentration of 5mmol / L~15mmol / L; ②Dissolve sodium periodate in distilled water to obtain a concentration of 0.11mol / L~0.33mol / L sodium periodate aqueous solution; ③, under the condition of avoiding light, mix the dextran aqueous solution with the concentration of 5mmol / L~15mmol / L and the concentration of 0.11mol / L~0.33mol / L L of sodium periodate aqueous solution was mixed, and stirred and reacted for 4h to 8h at a temperature of 25°C and a rotation speed of 800r / min to 1600r / min in the dark, to obtain a reaction product, which was transferred to a dialysis bag. The molecular weight cut-off of described dialysis bag is 3500, and de...
specific Embodiment approach 3
[0046] Embodiment 3: The difference between this embodiment and Embodiment 2 is that the average molecular weight of the dextran in Step ① is 10,000. Others are the same as in the second embodiment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com