Composition containing levorotatory amlodipine and azilsartan
A technology of levamlodipine and levamlodipine besylate, which is applied in the field of pharmaceutical preparations and can solve problems affecting the safety of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
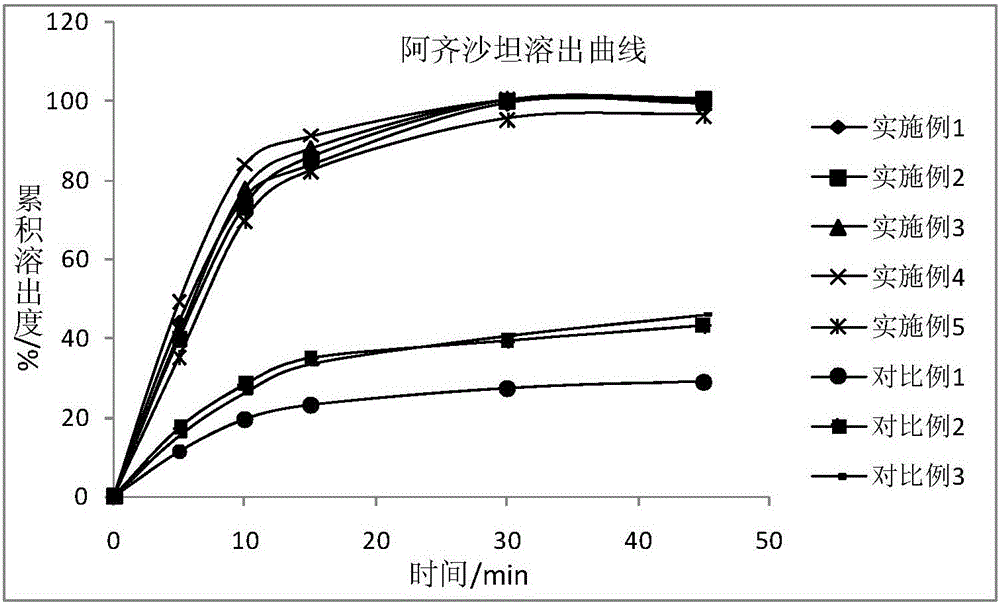
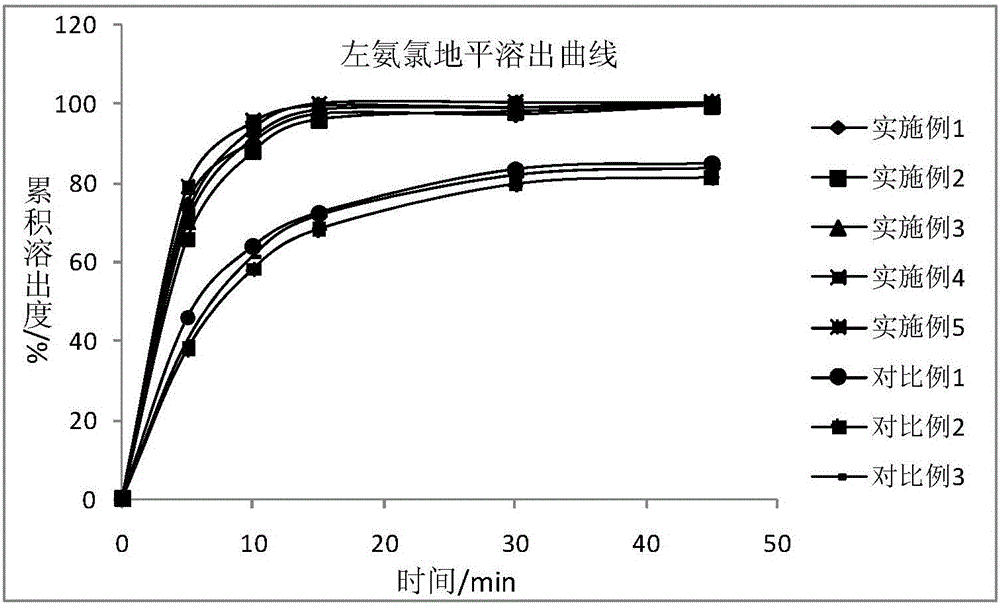
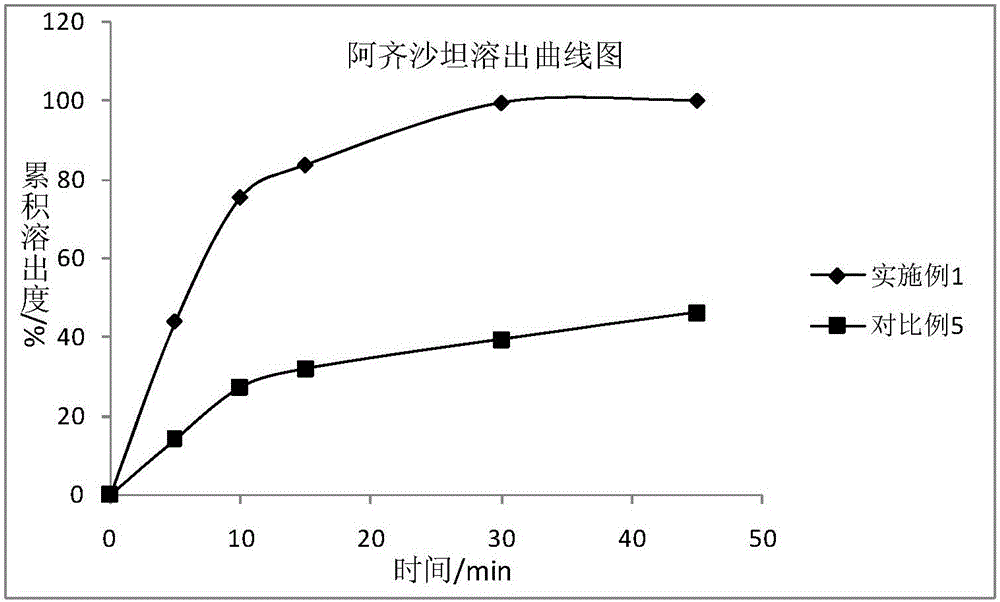
Image
Examples
Embodiment 6
[0051] Embodiment 6: Antihypertensive test in rats.
[0052] Experimental method: Take 120 healthy spontaneously hypertensive SHR rats, half male and half female, weighing 200-240g. Male and female rats are divided into 12 groups according to the balance of blood pressure (see Table 1 for specific grouping methods). For blood pressure, rat electronic sphygmomanometers were used to indirectly measure the systolic blood pressure of the rats when they were awake and quiet by the tail volume method. The grouping method is shown in Table 5.
[0053] model group
The same volume of 0.9% normal saline
/
Single drug 1 group
Levoamlodipine Besylate
5mg.kg -1 .d -1
Single drug 2 groups
20mg.kg -1 .d -1
Compound 1 group
Levoamlodipine Besylate / Azilsartan
2.5mg.kg -1 .d -1 / 10mg.kg -1 .d -1
[0054] Experimental results: Compared with the model group, the single drug 1 group, the single drug 2 grou...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com